Abstract
Neurothekeoma is a benign nerve sheath tumor, also known as nerve sheath myxoma. It arises from the cutaneous nerves of the head and neck region. In certain cases, neurothekeoma has been reported in the breast, oral cavity, tongue, maxilla, and spinal intradural space. Intracranial neurothekeoma, however, is an extremely rare entity, with only three cases reported in the literature: one in the parasellar region, one in the deep white matter, and another one in the cerebellopontine angle. We present the case of a 40-year-old man with a very large neurothekeoma present in the posterior fossa who had no neurologic deficit on presentation.
Introduction
Neurothekeoma is a benign nerve sheath tumor that arises from small cutaneous nerves and has a predilection for the upper part of the body: the head, neck, and shoulders.1,2 Sometimes it can be found in the breast,2 oral cavity,1 tongue,3 maxilla,4 and spinal intradural space.5,6 Neurothekeoma of the head and neck is quite common, with hundreds of reported cases in the literature.7 Intracranial neurothekeoma, however, is a very rare tumor, with only three cases reported in the literature, to our knowledge.8–10
We herein report a case of intracranial neurothekeoma, which had a unique location in the posterior fossa, mimicking a meningioma or a schwannoma.
Case Report
A 40-year-old man presented to the emergency room with a 2-week history of headaches. The patient complained of occipital headaches, which came on gradually and increased in intensity over the 2-week period. The day before the hospital admission, he also experienced transient numbness of the lower extremities. He did not have any difficulty ambulating or any balance problems. The patient had a history of type 2 diabetes, which was controlled with medications.
On neurologic examination, the patient had no cranial nerve deficits, no weakness, and no sensory deficits. He did not have dysmetria or dysdiadochokinesia. A head computed tomographic (CT) scan performed in the emergency room to evaluate for headache showed a left cerebellar mass. Thus, a magnetic resonance image (MRI) was obtained to better characterize the mass in the posterior fossa.
Imaging Findings
A noncontrast-enhanced CT scan of the head showed a mass in the left cerebellum with compression and distortion of the fourth ventricle. There was also evidence of ventriculomegaly.
Axial, coronal, and sagittal MRIs of the head displayed a round, well-delineated lesion in the posterior fossa compressing the left cerebellar hemisphere, with distortion of the brain stem and compression of the fourth ventricle. The tumor was hypointense to brain on T1-weighted images (Figure 1A) and hyperintense on T2-weighted images (Figure 1B). Fluid-attenuated inversion recovery images showed minimal edema around the tumor (Figure 1C). The tumor had faint enhancement with gadolinium administration, in a heterogeneous pattern (Figure 1D).
Figure 1.
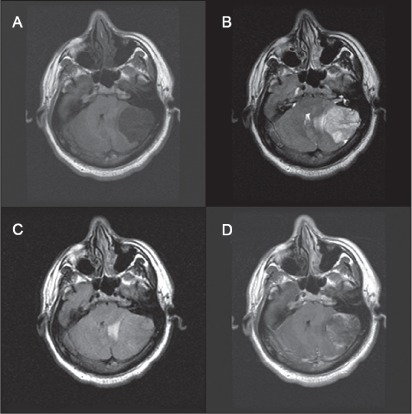
Axial magnetic resonance images of the tumor.
A) T1-weighted images show hypointense tumor. B) T2-weighted images show hyperintense lesion. C) Fluid-attenuated inversion images show minimal edema. D) T1-weighted images with contrast agent show heterogenous enhancement of the lesion.
To better characterize the lesion, an angiogram was obtained. Common carotid injections bilaterally showed no vascular abnormality. Vertebral injections showed filling of the basilar artery and the posterior circulation without a tumor blush. Right external carotid artery injection showed no abnormality. Left external carotid artery injection showed a faint vascular blush over the left cerebellar region. There was no distinct vessel supplying the mass. The occipital artery was cannulated, and injection of contrast showed small branches supplying the vascular blush, without evidence of a large vessel supplying the mass. Embolization was not performed.
Surgical Approach
The decision was made to proceed with surgery, for tissue biopsy and for attempted gross total resection. A standard left-sided posterior fossa craniotomy was performed. The dura mater was opened over the tumor, which had a well-demarcated capsule. The tumor was dissected carefully off the cerebellum. The tumor had areas that were soft and easily removable, mixed with areas of fibrotic tissue. It was quite avascular.
Pathologic Examination
On pathologic examination, the tumor had the appearance of a well-circumscribed, myxoid lobulated lesion. Histologically, the tumor was encapsulated by a thin fibrous connective tissue and was composed of ovoid lobules separated by fibrous septae. The lobules contained stellate and spindle-shaped cells with reticular cellular processes forming a myxoid network in an abundant basophilic matrix (Figure 2A, B). There was no necrosis and no mitoses (Figure 2A, B). The cells had a bland morphologic appearance despite cellular pleomorphism, as well as variable positivity for S100 immunohistochemical stain (Figure 2C) and negativity for glial fibrillary acidic protein stain (Figure 2D) characteristic of neurothekeoma.
Figure 2.
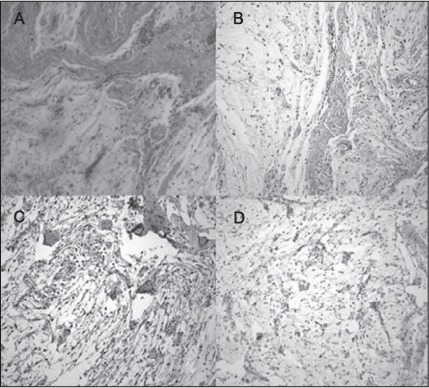
Pathologic examination of the tumor.
A) and B) Hematoxylin-eosin stain reveals hypocellularity. Cells are spindle shaped and surrounded by a myxoid matrix. C) Cells are positive for S100 antibody stain. D) Tumor cells stain negatively for glial fibrillary acidic protein.
Postoperative Course
Following complete total resection of the tumor, the patient had an uncomplicated postoperative course, without any neurologic deficits. Because of the benign nature of this tumor, the patient did not receive chemotherapy or radiation.
Discussion
Cutaneous neurothekeomas are classified into myxoid and cellular types. Both types of tumor are benign, and there have been no reported cases of metastases.7,11 Thus, radiation and chemotherapy are not recommended for treatment of cutaneous neurothekeoma.7,11 However, there are reported cases of recurrence. Although these cases were attributed to incomplete resection of the tumor,7,11 regular follow-up for surveillance is necessary to detect recurrence of these lesions.7,11
The most common type of intracranial neurothekeoma is myxoid. This tumor is characterized by hypocellularity, with small spindle or stellate cells loosely arranged in abundant mucinous stroma. On immunohistochemical staining, the tumor cells are positive for S100 antibody, nerve growth factor receptor (p75NGFR), collagen type IV, CD34, glial fibrillary acidic protein, and CD57.12
Our case initially was suspected of being a meningioma because of the appearance on MRI. The tumor was a well-circumscribed mass in the posterior fossa, located intradurally in an extra-axial location, and was pushing, rather than invading, the associated structures. The differential diagnosis for such a mass in the posterior fossa can also include schwannoma; other myxoid tumors such as sarcomas with myxoid degeneration, cardiac myxoma metastatic to the brain, primary intracranial myxoma, soft-tissue myxoma penetrating the skull; and gliomas.6,8,9
The most likely origin for the tumor presented in this case is the perineural cells of the nerves in the dura mater or around the blood vessels. Similar to cutaneous neurothekeomas, this intracranial tumor in the posterior fossa was not attached to a major cranial nerve. This made the resection easier and did not cause neurologic deficit for the patient. Cumulative experience from the literature regarding cutaneous neurothekeomas leads us to believe that intracranial neurothekeomas can be treated by gross total resection, with good outcomes, and do not require adjuvant chemotherapy or radiation.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
References
- 1.Vered M, Fridman E, Carpenter WM, Buchner A. Classic neurothekeoma (nerve sheath myxoma) and cellular neurothekeoma of the oral mucosa: immunohistochemical profiles. J Oral Pathol Med. 2011 Feb;40(2):174–80. doi: 10.1111/j.1600-0714.2010.00952.x. [DOI] [PubMed] [Google Scholar]
- 2.Wee A, Tan CE, Raju GC. Nerve sheath myxoma of the breast. A light and electron microscopic, histochemical and immunohisto-chemical study. Virchows Arch A Pathol Anat Histopathol. 1989;416(2):163–7. doi: 10.1007/BF01606322. [DOI] [PubMed] [Google Scholar]
- 3.Makino T, Utsunomiya T, Kamino Y, et al. Nerve sheath myxoma of the tongue in a child. Int J Oral Maxillofac Surg. 2002 Aug;31(4):451–4. doi: 10.1054/ijom.2001.0202. [DOI] [PubMed] [Google Scholar]
- 4.Cohen NA, Samadi DS, Pawel BR, Kazahaya K. Cellular neurothekeoma of the maxilla. Ann Otol Rhinol Laryngol. 2004 May;113(5):384–7. doi: 10.1177/000348940411300508. [DOI] [PubMed] [Google Scholar]
- 5.Lee D, Suh YL, Han J, Kim ES. Spinal nerve sheath myxoma (neurothekeoma) Pathol Int. 2006 Mar;56(3):144–9. doi: 10.1111/j.1440-1827.2006.01933.x. [DOI] [PubMed] [Google Scholar]
- 6.Paulus W, Jellinger K, Perneczky G. Intraspinal neurothekeoma (nerve sheath myxoma). A report of two cases. Am J Clin Pathol. 1991 Apr;95(4):511–6. doi: 10.1093/ajcp/95.4.511. [DOI] [PubMed] [Google Scholar]
- 7.Hornick JL, Fletcher CD. Cellular neurothekeoma: detailed characterization in a series of 133 cases. Am J Surg Pathol. 2007 Mar;31(3):329–40. doi: 10.1097/01.pas.0000213360.03133.89. [DOI] [PubMed] [Google Scholar]
- 8.Paulus W, Warmuth-Metz M, Sörensen N. Intracranial neurothekeoma (nerve-sheath myxoma). Case report. J Neurosurg. 1993 Aug;79(2):280–2. doi: 10.3171/jns.1993.79.2.0280. [DOI] [PubMed] [Google Scholar]
- 9.Pal L, Bansal K, Behari S, et al. Intracranial neurothekeoma— a rare parenchymal nerve sheath myxoma of the middle cranial fossa. Clin Neuropathol. 2002 Mar–Apr;21(2):47–51. [PubMed] [Google Scholar]
- 10.Erdem Y, Koktekir E, Bayar MA, Yilmaz A, Caydere M. Characterization of an intracranial neurothekeoma: case report. Turk Neurosurg. 2012;22(1):109–12. doi: 10.5137/1019-5149.JTN.3099-10.2. [DOI] [PubMed] [Google Scholar]
- 11.Fetsch JF, Laskin WB, Hallman JR, Lupton GP, Miettinen M. Neurothekeoma: an analysis of 178 tumors with detailed immunohistochemical data and long-term patient follow-up information. Am J Surg Pathol. 2007 Jul;31(7):1103–14. doi: 10.1097/PAS.0b013e31802d96af. [DOI] [PubMed] [Google Scholar]
- 12.Laskin WB, Fetsch JF, Miettinen M. The “neurothekeoma” : immunohistochemical analysis distinguishes the true nerve sheath myxoma from its mimics. Hum Pathol. 2000 Oct;31(10):1230–41. doi: 10.1053/hupa.2000.18474. [DOI] [PubMed] [Google Scholar]


