Abstract
Drosophila has been established as an excellent genetic and genomic model to investigate host-pathogen interactions and innate immune defense mechanisms. To date, most information on the Drosophila immune response derives from studies that involve bacterial, fungal or viral pathogens. However, immune reactions to insect parasitic nematodes are still not well characterized. The nematodes Heterorhabditis bacteriophora live in symbiosis with the entomopathogenic bacteria Photorhabdus luminescens, and they are able to invade and kill insects. Interestingly, Heterorhabditis nematodes are viable in the absence of Photorhabdus. Techniques for infecting Drosophila larvae with these nematodes have been previously reported. Here, we have developed a method for infecting Drosophila adult flies with Heterorhabditis nematodes carrying (symbiotic worms) or lacking (axenic worms) their associated bacteria. The protocol we present can be readily adapted for studying parasitic strategies of other insect nematodes using Drosophila as the host infection model.
Keywords: Drosophila, Heterorhabditis, Photorhabdus, infection model, model host, nematode parasitism
Introduction
The use of insect models to investigate pathogenic infection processes and host innate immune mechanisms offers certain advantages over the use of mammalian models. Insects are easy to maintain in large quantities and their small size facilitates artificial infections and extractions of tissues. Insects also share many physiological processes with mammalian hosts, and they can be used as efficient models to discover toxins and virulence factors some of which are also required to subvert mammalian defenses.1-6
Drosophila melanogaster has been established as an ideal model for studying host-pathogen interactions, as it benefits from the development of a wide range of molecular and cellular tools, impressive advances in the application of high throughput forward and reverse genetic/genomic screens and the body of knowledge accumulated by thousands of investigators.7 In addition, innate immunity in Drosophila can be studied as an integrated system at the level of the whole organism.8 In recent years, genetic studies in Drosophila have resulted in the discovery of distinct immune signaling pathways in response to microbial infections.9 These advances have enjoyed a high profile because they have produced the major spin-off of rekindling interest in the innate immune system of mammals. Largely thanks to work done first on Drosophila, it is now recognized that the nuclear localization of nuclear factor kappa B (NFκB) transcription factors is a universal feature of innate immune reactions,10,11 and that Toll-like receptors are of outstanding importance in transducing innate immune responses in vertebrates.12-15
Drosophila has a multilayered immune response consisting of humoral and cellular mechanisms.16 The hallmark of the Drosophila host defense is the definition of two main signaling pathways, Toll and immune deficiency (Imd), which lead to the activation of distinct members of the NFκB family of transcription factors, and result in the expression of hundreds of target genes, including those encoding antimicrobial peptides (AMPs).17-19 Activation of these pathways depends on recognition of certain microbial elicitors such as bacterial peptidoglycans and fungal glucans.20,21 Two other signaling pathways, the c-Jun N-terminal kinase (JNK) and Janus kinase-signal transducer and activator of transcription (JAK-STAT), also participate in regulating immunity effector genes in Drosophila.22,23 The body cavity of Drosophila, like that of all arthropods, is filled with a circulating hemolymph that contains both free-floating and sessile blood cells (hemocytes). These are responsible for a number of cellular defenses, while they can take part in humoral reactions.24,25 In addition, Drosophila can activate complex proteolytic cascades that regulate coagulation and melanization of hemolymph,26,27 defenses associated with the production of reactive oxygen and nitrogen species,28,29 and epithelial responses in the gut that also play important roles in fighting microbial infections.8,30
Drosophila has previously been used as a model host to investigate immune responses to diverse pathogenic organisms, including bacteria, fungi, viruses and parasitoid wasps.9,20 Recent work has also begun to use the power of Drosophila to dissect the molecular basis of the insect immune response to the combined insult of insect parasitic nematodes and their mutualistic bacterial pathogens.31-34 Unlike many animals associated with bacterial symbionts, entomopathogenic nematodes are viable in the absence of their mutualistic bacteria.35 Consequently, each partner of the mutualistic relationship can be separated and studied in isolation or in combination, thus enabling pathogenesis to be studied individually or together.36
In addition, to being highly virulent parasites of insects, Heterorhabditis bacteriophora nematodes maintain a mutualistic relationship with the entomopathogenic bacteria Photorhabdus luminescens.37 In particular, the bacteria are found in the gut of infective juvenile (IJ) worms that are able to attack and invade insects.38 Once inside the insect, the IJ regurgitates Photorhabdus into the hemolymph where the bacteria begin to divide exponentially producing a wide range of toxins and hydrolytic enzymes that result in insect death.39 At the same time, the IJ exits diapause and develops into an adult hermaphrodite nematode in a process called IJ recovery. The adult hermaphrodite lays eggs that hatch and develop through four juvenile stages into adult nematodes. Remarkably a single IJ entering an individual insect will result in the production of > 100,000 IJs over a time-scale of 2–3 weeks. This extremely efficient relationship provides a fascinating model system for studying bacterial pathogen, nematode-vector and insect host interactions.40-44
Protocols for infecting Drosophila larvae with Heterorhabditis nematodes have recently been published;31-33 however, it is not currently known how Drosophila adults respond to nematode infection. Interestingly, differences have been found between the adult fly and larval immune systems that are probably due to physiological differences between larvae and adults, and differences in their lifestyles.45-48 Here we report the development of a new assay for infecting adult flies with axenic and symbiotic worms (Fig. 1). We have used this method to monitor fly survival and nematode load and show that Heterorhabditis nematodes are able to develop in Drosophila adults. In future studies, we will employ this infection protocol to assess immune gene expression in nematode infected wild-type (WT) and mutant adult flies in order to elucidate how parasitic infections are sensed, and how their presence is communicated both within and among cells and tissues of the host.
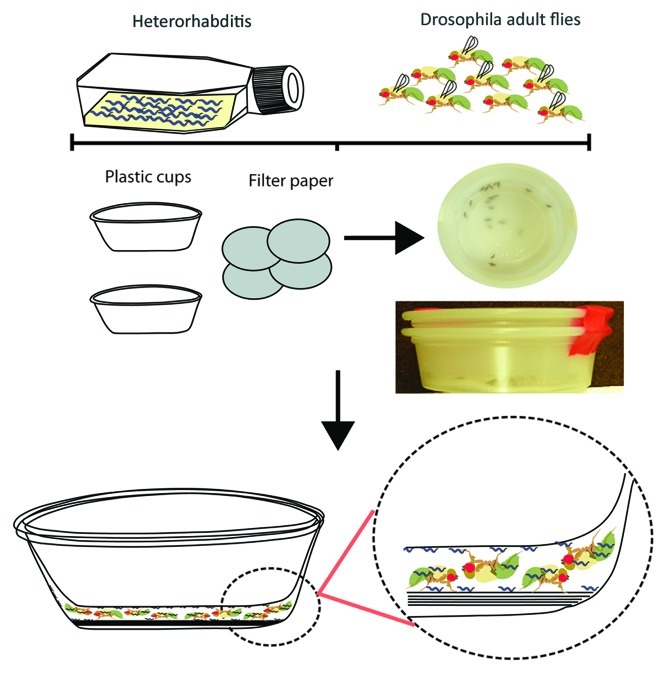
Figure 1. Outline of the method for infecting Drosophila adult flies with Heterorhabditis nematodes. We use 4–6 d old adult flies in the infection experiments. Nematode infective juveniles (IJ) are kept in sterile water in tissue culture flasks. Filter paper discs are transferred to the bottom of small plastic cups. Approximately 1,000 nematodes are pipetted onto the filter papers and 10 flies are transferred to each test cup. A second identical plastic cup is inserted into the test cup to restrict movement of flies between the filter papers and the bottom of the inserted cup.
Experimental Design
Fly strain
We used 4–6 d old adult flies of the Drosophila strain OregonR. Both male and female flies were used in the assays. Flies were reared at relatively low densities (20 individuals per vial) to prevent overcrowding conditions. All experiments were performed using flies from the same batch, whenever possible, to reduce variation.
Bacterial/nematode strains
Heterorhabditis bacteriophora nematodes and Photorhabdus luminescens subsp laumondii strain TT01 bacteria were used in all experiments. We also used 2–4 weeks old Heterorhabditis IJ, as newly hatched and old nematodes show reduced infectivity and host seeking behavior (unpublished data). To generate axenic nematodes, we used bacteria of the Photorhabdus temperata mutant strain RET16 that support the growth of Heterorhabditis without colonizing the nematodes.31 Nematodes were cultured on lipid agar plates supplemented with carbenicillin and gentamicin. This resulted in selection of Photorhabdus luminescens cells that were obtained by the worms. Consequently, nematodes fed on RET16 bacteria turn to axenic (Photorhabdus-free) once they complete their life cycle.
Materials
Organisms
Insects
OregonR adult flies (kindly provided by Prof. J.M. Reichhart, University of Strasbourg and CNRS, Strasbourg, France); Galleria mellonella sixth instar larvae (PetCo).
Nematode species
Heterorhabditis bacteriophora strain TT01.
Bacterial strains
(a) Photorhabdus luminescens subsp laumondii strain TT01 (kindly provided by Dr David Clarke, University College Cork, Cork, Ireland)
(b) RET-16 mutant, a GFP-labeled variant of Photorhabdus temperata strain NC1 (gentamicin resistant) (kindly provided by Dr Todd Ciche, Michigan State University)
(c) Escherichia coli (streptomycin resistant) (ATCC #25254), kindly provided by Prof. Michael R. Strand, University of Georgia).
Reagents
Luria-Bertani (LB) broth (Miller) (1% tryptone, 0.5% yeast extract, 1% NaCl) (Amresco, J106)
Bacteriological agar (Amresco, J637)
Corn Syrup (MP Biochemicals, 101413)
Cod liver oil (MP Biochemicals, 901405)
0.5M magnesium chloride solution (Acros organics, 7791-18-6)
Gentamicin (stock solution 10 mg/mL) (VWR, 97061-370)
Carbenicillin (stock solution 100 mg/mL) (VWR, 97063-146)
Phosphate-buffered saline (PBS) (Fisher Scientific, BP399-500)
Sterile water: autoclaved distilled water
1% Bleach solution for nematode sterilization
10% Bleach solution for cleaning surfaces
Ethanol (Absolute), 70% solution for surface sterilization (Decon-Labs, 2716)
Active dry yeast (Fisher Scientific, S802452)
Instant Drosophila media, Equation 4-24 (Carolina Biol. Supply Co., 173202)
0.85% Sodium chloride solution (Fisher Scientific, BP358-1)
Glycerol (Fisher Scientific, 633-500)
Mineral oil (Alpha Aesar, 8020-83-5)
Equipment
Fly incubators (25°C temperature, 60–75% humidity, 12-h light/12-h dark photoperiod.
Paintbrush (size 0) (Fisher Scientific)
General-purpose microbiological incubator (VWR)
Petri dishes (150 × 15 mm) (VWR, 25384-326)
Petri dishes (100 × 15 mm) (VWR, 25384-342)
Compartmentalized Petri dishes (Bi-plates) (100 × 15 mm, 2 sections) (Fisher Scientific, 0875150)
Whatman filter paper, Grade 1 (90 mm diameter) (Whatman, 1001090)
Whatman filter paper, Grade 1 (150 mm diameter) (Whatman, 1001150)
Graduated disposable pipettes (25 mL) (Corning)
One liter bottles (Corning)
96 well plates (Corning)
Microscopy glass slides (Fisher Scientific, 12-550-15)
Glass coverslips (60 × 24 mm) (Fisher Scientific, 12-545-M)
Cell culture flasks, 80 cm2 culture area (Thermo, 153732)
Cell culture flasks, 175 cm2 culture area (Thermo, 156502)
Plastic cups 0.5 Oz (Solo Cup Co., P050-0100)
15 mL conical polypropylene tubes (VWR, 21008-103)
50 mL conical polypropylene tubes (Falcon, 352098)
Kimwipes (4 × 8 in) (Fisher Scientific, S47299)
Orbital shaker (New Brunswick)
Needle puller P-1000 (Sutter Instruments)
Borosilicate capillary needles, Sutter Instruments (OD: 1.0 mm, ID: 0.50 mm, B100-50-10)
Centrifuge with interchangeable rotors for 1.5 mL microcentrifuge tubes and 15, 50 mL conical tubes (Eppendorf)
Stereomicroscope (Tri-Tech)
Nanoject II (Drummond)
10 mL disposable syringe (BD Medical, 14-823-2A)
25 G syringe needle (Fisher Scientific, BDC-5124)
0.22 µm syringe filter sterile (Fisher Scientific, 09-719C)
Color tape (Fisher Scientific, 15-901-15A)
1.5 mL microcentrifuge tubes (US Scientific, 1615-5510)
Nanodrop spectrophotometer (Thermo Scientific)
Disposable spectrophotometer cuvettes (VWR, Cat. No. 89005-758)
Micropipettes (10, 100, 200, 1000 µL) (Rainin)
Drosophila vials (VWR, 734-2255)
Disposable bacterial spreaders (VWR, 89042-018)
Drummond #5 Forceps (Fine science tools, 11252-40)
Pneumatic pump for pipettes (VWR, 53502-211)
Water bath, Isotemp 215 (Fisher Scientific)
Graduated 100 mL cylinder (Fisher Scientific)
Carbon dioxide (CO2) anesthetizing system for Drosophila (Genesee Scientific)
Rubbermaid container, 6.5 qt. (Rubbermaid, 3Q31)
UV Transilluminator, Spectrolinker XL-1000 (Spectronics corporation)
Setup of reagents
Drosophila food preparation
Prepare Drosophila instant media according to the manufacturer’s instructions. Briefly, take one small scoop of media and add it to a Drosophila vial. Add 10 mL of sterile water and let the mix sit for 2 min; add 5–10 yeast granules and cover the tubes with cotton balls. Label accordingly. Always use fresh vials when preparing fly cultures.
Preparation of antibiotic stocks
Prepare stock solutions of gentamicin (10 mg/mL) and carbenicillin (100 mg/mL) by diluting the antibiotics in sterile water and filtering the solution through a 0.2 µm syringe filter. Prepare rifampicin stock solution (75 mg/mL) by dissolving the antibiotic in DMSO and vigorous vortexing (no sterilization is required). Keep antibiotic stocks at -20°C in the dark.
Preparation of lipid agar plates
They are used for growing axenic nematodes. Add 25 g of LB broth, 17 g of bacteriological agar, 10 mL of corn syrup in 1 L of double distilled water and autoclave the solution. After autoclaving, keep the solution at 60°C in a water bath. Add 5 mL of cod liver oil (autoclaved), 1 mL of 0.5 M MgCl2, and carbenicillin, gentamicin antibiotics (final concentration in water: 100 µg/mL and 0.75 µg/mL, respectively). Mix well and pour into one side of a compartmentalized-plate. The plates are wrapped in aluminum foil and stored at 4°C until needed.
Preparation of LB agar plates
These are used for culturing bacteria. Plain LB agar plates are prepared by mixing 25 g of LB, 17 g of agar in 1 L of double distilled water and the solution is autoclaved. A separate batch of LB agar plates supplemented with rifampicin (75 µg/mL) is prepared to test for the presence of Photorhabdus bacteria in axenic worms. Rifampicin plates should be kept in the dark. All plates are stored at 4°C.
Counting nematodes
Agitate a flask containing nematodes in suspension and pipette ten individual drops (aliquots) of 50 µL each of water-containing nematodes on a Petri dish; count the number of worms in each drop using a stereomicroscope. Calculate the total number of worms by adding all ten counts and multiply by 20 to obtain X number of nematodes per mL. Use the number of nematodes/mL to calculate doses.
Precautions
(1) All surfaces should be sterilized with 10% bleach to avoid contaminations.
(2) Forceps and brushes that are used to manipulate flies should be sterilized for 6 min using an UV trans-illuminator. Alternatively, forceps should be cleaned with 70% ethanol.
(3) Add water to bi-plates under sterile conditions.
(4) All contaminated materials should be discarded in a biohazard container for proper disposal.
(5) All solutions should be autoclaved unless otherwise indicated and antibiotics should always be filter sterilized.
(6) Wear gloves at all times.
Procedure
Culturing symbiotic nematodes: Timing 14–20 d
To grow and amplify Heterorhabditis symbiotic nematodes, we use caterpillars of the greater wax moth Galleria mellonella. The larvae are infected with nematodes carrying P. luminescens strain TT01 bacteria.
(1) Plate preparation: 1 h
Cover the bottom of a large Petri dish (150 mm diameter) with a single filter paper, add 1 mL of sterile water and transfer 6–10 Galleria larvae. Pipette a drop of water containing 50–100 IJ on each insect.
(2) Incubation: 11 d
Galleria larvae succumb to nematodes 2 d after the infection. Heterorhabditis/Photorhabdus infected Galleria larvae exhibit a characteristic “red brick” color (Fig. 2A). Insects turning black or gray are contaminated by other pathogens and they should be immediately discarded. Monitor the survival of larvae daily and add a few drops of water, if needed, to keep the insect cadavers moist but not soaked.

Figure 2. Water trap setup. (A) Galleria mellonella cadavers are arranged on top of the filter paper (note the characteristic “red brick” color of nematode infected larvae). Aerial (Top) and lateral (bottom) views showing the insect larvae arranged on top of the cut filter paper centered in the Petri dish. Note the arrangement of the small Petri dish, centered in the large Petri dish, the position of the filter paper and the edge of the paper making direct contact with the water. (B) Filter papers are folded and cut in the corners to create “projections” that will be in contact with the water. This facilitates the transfer of moisture and the migration of Heterorhabditis IJ from the insect cadavers to the water trap.
(3) Water trap preparation: 1 h
On day 11 after infection, transfer the Galleria larvae to a water trap. This consists of a large Petri dish (150 × 20 mm), inside of which a smaller standard size Petri dish (100 × 15 mm) is placed upside down. We place a piece of filter paper (cut as shown on Fig. 2B) on top of the small Petri dish and fill the large Petri dish with water until the edges of the filter paper are submerged.
(4) Insect cadavers as prey for nematodes: 10 min
Using a pair of plastic forceps, gently transfer and arrange the infected Galleria larvae on the filter paper of the water trap. Place the traps in a Rubbermaid container to prevent contamination of the cadavers by other pathogens. Store in the dark until the IJ emerge from the insect cadavers.
(5) Nematode harvesting: 30 min
Nematodes usually emerge from the dead insects 14–20 d after infection. Emerging nematodes directly fall into the water allowing for easy collection. They can be readily collected from the water trap using a 100 mL graduated cylinder. Fill the cylinder to the top (100 mL mark) with water containing nematodes and let the worms settle at the bottom (this takes approximately 30 min). Remove 85 mL of water (down to the 15 mL mark) using a volumetric pipette. Fill back up with fresh water to the 100 mL mark, let the nematodes settle, and remove the excess water as indicated above. Repeat three times until the water in the cylinder is clear. Transfer 20 mL aliquots in small culture flasks, and store in the dark. Nematodes can be collected daily for at least 2 weeks or longer depending on the number of worms required for experiments.
NOTE: It is important to achieve optimum numbers of nematodes in each flask: high density will result in nematode aggregations and eventually nematode death; low density will not produce the appropriate number of worms required for the experiments. We keep around 1,500–2,000 IJ/mL of water. Shake flasks regularly to aid aeration. Nematodes can be stored at room temperature (RT) (~25°C) for months. We always use less than 1-mo-old nematodes in our experiments.
Culturing axenic nematodes: timing 14–20 d
Axenic nematodes (nematodes lacking Photorhabdus bacteria) are cultured in bi-plates on a bacterial lawn produced by P. temperata mutant strain RET16.
(6) Bacterial inoculation and plating: 18 h
Grow P. temperata RET16 bacteria in LB broth at 30°C with vigorous agitation for 18 h; transfer 500 µL of pure culture to LB agar bi-plates supplemented with 100 µg/mL of carbenicillin and 0.75 µg/mL of gentamicin. Incubate plates for 48 h at 30°C to form a bacterial lawn.
(7) Incubation of nematodes mixed with bacterial lawn: 30 min–12 d
Transfer 500–1,000 surface-sterilized IJ to the center of the bacterial lawn; gently agitate plates to distribute the worms evenly and incubate at 30°C for 12 d.
NOTE: A container filled with sterile water is placed in the incubator to prevent desiccation. Sterile water (approximately 400 µL) is also added to the plates after 1 week (or whenever necessary), to moisten the agar.
(8) Water trap setup: 10 min
After 12 d of incubation, fill the other half of the bi-plate with sterile water and incubate at RT to allow emerging IJ to fall into the water trap as they migrate from the agar to the water.
(9) Nematode collection: 1 h
After 14–20 d, IJ nematodes are collected from the water traps. Aspirate the water using a 10 mL disposable pipette and replace with fresh water. Transfer the water containing nematodes to a culture flask. Wash nematodes several times with sterile water and store flasks in the dark. Nematodes should always be surface sterilized before use, as described above.
(10) Testing axenicity: 2–3 d
To determine whether axenic Heterorhabditis contain Photorhabdus cells, pellet a sample of newly generated axenic nematodes (200 µL of a nematode suspension in a 1.5 mL tube) for 5 min at 8,000 rpm. Remove the supernatant and homogenize the worms using a plastic pestle. Add 100 µL of LB broth, mix and plate out the solution on LB agar plates supplemented with rifampicin (see above); incubate for 2 d at 30°C. The absence of bacterial colonies indicates that no P. luminescens cells are present in the worms.
Surface sterilization of nematodes: timing 1 h
(11) Pelleting nematodes: 10–15 min
Centrifuge 20 mL of a nematode suspension in a 50 mL conical tube at 1,500 rpm (standard speed used in all steps) for 5 min; discard the supernatant.
(12) Bleach treatment: 10–15 min
Resuspend the nematode pellet in 20 mL of 0.1% bleach solution in water, gently invert the tube several times, incubate for 7 min at RT and centrifuge for 5 min at 1,500 rpm. Remove the bleach solution leaving the pellet in approximately 1 mL of solution left, add fresh water (40 mL), mix gently and repeat centrifugation step. Repeat four times. Nematodes are resistant to low concentrations of bleach. We did not observe any negative effects of bleach on the fitness or survival of the nematodes.
(13) Washes: 25 min
Add 20 mL of 1x PBS, invert the tube three times and centrifuge for 5 min. Repeat this step three times. Finally, the nematodes are resuspended in sterile water or 0.85% of NaCl and stored in sterile flasks until needed.
Presence of bacteria in infected flies (survival experiment controls): timing 20 h
Positive and negative controls in the survival experiments include flies injected with pathogenic P. luminescens and non-pathogenic E. coli bacteria, respectively.
(14) Bacterial growth: 18 h
Inoculate bacteria from frozen glycerol stocks in 10 mL of LB broth and incubate with constant agitation (250 rpm) for 18 h at 30°C for P. luminescens and 37°C for E. coli.
(15) Wash bacteria: 45 min
Centrifuge bacterial cultures for 5 min at 5,000 rpm to pellet the bacterial cells; discard the supernatant. Wash the pellet twice using sterile 1x PBS and centrifuge for 5 min at 5,000 rpm; resuspend the pellet in 7 mL of 1x PBS. Measure the optical density (OD) using a spectrophotometer. Adjust the concentration at an OD of approximately 0.1 to obtain adequate cell density.
Bacterial injection (survival experiment controls): 1–2 h
We use a nanoinjector and glass capillary needles for delivering precise doses of bacteria into flies.
(16) Preparation of capillary needles: 10 min
Secure a borosilicate needle to the pipette puller and press the “Pull” button. Pipette puller settings are: ramp: 561, velocity: 20, heat: 560, delay: 1, pull: 100, pressure: 500. The needle tip is manually broken under the stereoscope using a pair of clean forceps. The capillary is then filled with mineral oil using a plastic syringe and 25G gauge needle.
(17) Injector assembly: 3 min
Attach the needle to the injector by connecting all o-rings and securing the cap tightly. Pipette 50 µL of washed bacterial cells of the appropriate concentration on a piece of parafilm. Fill the needle by pressing the “Fill” button.
(18) Injections: 1.5–2 h
Place the flies on a fly pad and anesthetize them with CO2; flies are arranged with a paintbrush and injected with 18.6 nl of a bacterial suspension corresponding to an OD of 0.1 (approximately 400–700 CFUs for P. luminescens and 1,000–4,000 CFUs for E. coli). Transfer infected flies to fresh vials and monitor their survival daily and up to 7 d.
Nematode infection assays: 5–7 d
(19) Preparation of infection units: 45 min–1 h
Cover the bottom of the cups with 4–5 pieces of filter paper; use the bottom of the cup as measurement.
(20) Mixing nematodes and flies: 15 min
Surface sterilized nematodes in solution (100 IJ/fly) are pipetted to each infection unit. Flatten the filter paper at the bottom of the cup using a micropipette tip. Add more water to the filter paper as needed (usually in aliquots of 100 µL) until it is attached to the bottom of the cup; this step is important because extra water keeps the nematode parasites active.
Tip: For four layers of filter paper, the maximum total volume of added water is approximately 600 µL; for five filter papers it is about 800 µL. Once nematodes and flies are added to the cup, a second empty cup is placed on top and secured using tape (Fig. 1). Keep the infection units at RT in a humid box (e.g., a pipette tip box lined with wet filter paper).
NOTE: It is important to prevent desiccation. The addition of extra water to the cups depends on nematode density: the higher the density, the more water needs to be added, and vice versa. Excess water will result in fly drowning; lack of water will result in desiccation and nematode death. It is also important to flatten the filter papers at the bottom of the cup to avoid trapping of flies in between the filter papers. Cups should be prepared 15–30 min before use.
Survival experiments: 7 d
(21) Set up: 30 min
Infect 4–6 d old OregonR adult flies with 100 IJ/fly as described above.
(22) Monitoring survival: 7 d
Count and record the number of dead flies daily.
Dose-response survival experiments: 7 d
(23) Set up: 30–45 min
Infect 4–6 d old OregonR adult flies with 10, 25, 50 and 100 nematode IJ/fly. Estimate nematode numbers as described above.
(24) Monitoring survival: 7 d
Incubate infected and uninfected control flies for 7 d at RT and count the number of dead flies daily.
Persistence experiments: 2 d
(25) Set up: 30 min
Infect 4–6 d old OregonR adult flies with 100 IJ/fly as described above. Incubate flies at RT.
(26) Nematode counting: 2 d
At 24 and 48 h post-infection, wash infected (dead or alive) flies in a drop of 1x PBS to remove any nematodes attached to the exterior of the flies (use the microscope). Dissect flies in 1x PBS using a pair of clean forceps to determine the number of nematodes present in each fly.
Nematode development: 5 d
To determine whether Drosophila adult flies are suitable hosts for supporting the development of Heterorhabditis nematodes, we infect flies with symbiotic nematodes (50 IJ/fly), as described above. We have observed that infection with a high number of nematodes slows down nematode development, probably due to crowding conditions in the fly.
(27) Set up: 15 min
Infect Drosophila adult flies as previously described and incubate insects at RT.
(28) Dissection and fixation: 1–2 h
Five days after infection, dissect flies in sterile 1x PBS. Incubate dissected cadavers in cold 4% paraformaldehyde diluted in 1x PBS, in a 96-well plate for 1 h. This treatment eliminates any remaining live nematodes. Gently remove the fixative solution and wash samples three times in 1x PBS. Carefully mount the dissected flies on a glass slide using 50% glycerol diluted in 1x PBS solution, and cover the samples with a coverslip. Specimens are ready for viewing under the microscope. Slides can be stored at 4°C for a few days.
(29) Imaging: flexible
Time needed for viewing depends on the number of prepared samples and experience of the researcher with microscopy techniques. Images are assembled and illustrations prepared using Adobe Illustrator and Photoshop software packages (Adobe CS5 suite).
Results and Further Studies
We first infected Drosophila WT adult flies with Heterorhabditis nematodes carrying or lacking Photorhabdus bacteria and estimated the ability of flies to survive infection over time (Fig. 3). We found that in both cases flies succumbed to nematode infection after 5–6 d. Survival of flies infected with axenic or symbiotic nematodes was highly significant (p < 0.05; Chi-square test) compared with uninfected controls. High fly mortality caused by axenic nematodes merits further investigation and it will be the focus of our future studies. Injection of Drosophila with Photorhabdus (positive control) also killed all flies within the same length of time. Injection of flies with E. coli bacteria or uninfected flies (negative controls) produced minimum insect mortality.

Figure 3. Survival results after infection of Drosophila wild-type (WT) adult flies with axenic Heterorhabditis (nematodes lacking Photorhabdus), symbiotic Heterorhabditis (nematodes carrying Photorhabdus) or Photorhabdus bacteria alone. Each replicate consisted of 10 Oregon 4–6-d-old flies infected with approximately 1,000 nematodes (100 IJ per fly). Flies were injected with 18.4 nl of PBS buffer containing approximately 500 Photorhabdus cells. Infections with E. coli bacteria (500 cells) and injections with PBS buffer alone served as controls. Results showed that both axenic and symbiotic nematodes were able to kill adult flies at a similar rate. Survival of flies was monitored daily and up to day six following infection. Data represent the percent survival of infected and control flies. The averages from at least three experiments are shown.
We then infected Drosophila adult flies with different numbers of axenic or symbiotic Heterorhabditis IJ. Interestingly, we found that flies infected with 25 or 100 axenic Heterorhabditis died slightly faster (3 d after infection) compared with those infected with 10 or 50 axenic worms (4 d after infection) (Fig. 4A). Different number of axenic nematodes caused mortality that was significantly higher compared with uninfected controls (p < 0.05; Dunnett’s multiple comparison test). One explanation for this discrepancy could be variation in nematode seeking behavior or increased fly activity. We have found that hyperactive flies significantly lower Heterorhabditis infectivity (unpublished data). However, we observed that all flies infected with different numbers of symbiotic Heterorhabditis succumbed within 4 d of infec>tion (Fig. 4B). Again, mortality of uninfected control flies was significantly lower (p < 0.05; Dunnett’s multiple comparison test) compared with mortality of flies infected with symbiotic nematodes.
Figure 4. Dose-response survival results for Drosophila wild-type (WT) adult flies infected with (A) axenic Heterorhabditis (nematodes lacking Photorhabdus) and (B) symbiotic Heterorhabditis (nematodes carrying Photorhabdus). We used 10, 25, 50 and 100 IJ per fly. Ten Oregon 4–6-d-old flies were used for each treatment. Uninfected flies served as controls. Survival of flies was estimated daily and up to day seven following infection. Data represent the percent survival of infected and control flies. The averages from at least three experiments are shown.
We further looked at the persistence of axenic and symbiotic Heterorhabditis in Drosophila WT flies at 24 and 48 h after infection (Fig. 5). We found that both groups of nematodes were present in flies at both time-points, which suggests that Drosophila adults can serve as valuable model for studying host-parasite interactions and nematode parasitism. We did not observe any significant difference in the number of axenic or symbiotic worms between the two time-points (p < 0.5, df = 3; one-way ANOVA)

Figure 5. Persistence of axenic and symbiotic Heterorhabditis in Drosophila adult flies. Ten Oregon 4–6-d-old flies were used in each replicate. Flies were infected with approximately 1,000 nematodes (100 nematodes per fly), and dissected at 24 and 48 h after infection. Nematode numbers were counted under a dissecting microscope. Colored horizontal lines indicate the arithmetic means of nematode numbers and error bars indicate standard errors. Data represent the averages from at least three independent experiments.
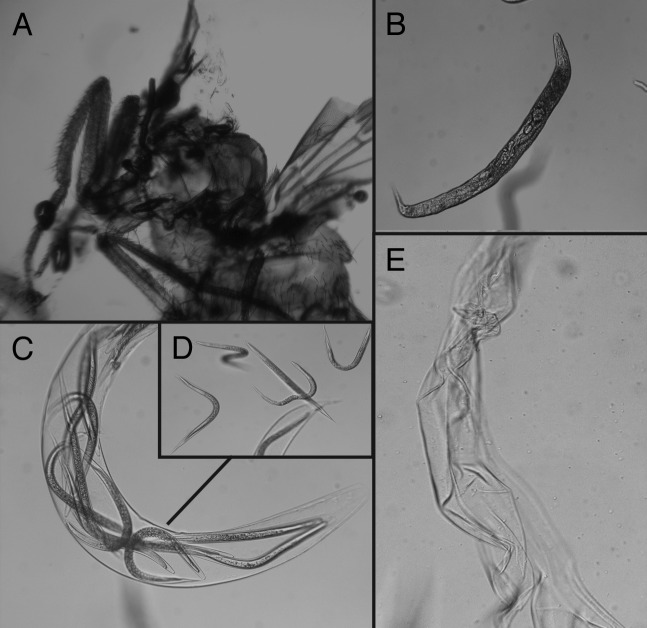
Finally, we asked whether Drosophila adult flies permit nematode development. For this, we used Heterorhabditis IJ to infect WT flies that were dissected 5 d later (Fig. 6A). We found that infected flies contained nematodes at different stages of development; these included pre-hermaphrodite nematodes (Fig. 6B), hermaphrodites harboring newly hatched L1 nematodes (Fig. 6C), and pre-IJ worms that disrupt the nematode cuticle and move to the fly hemocoel (Fig. 6D). We also observed cuticles of hermaphrodites (or mother worms) that were left empty after the new generation of nematodes had escaped to the insect body cavity (Fig. 6E). The various stages of nematode life cycle were not observed outside flies. These results suggest that Drosophila adult flies, despite their small size, are able to support the development of Heterorhabditis nematodes.
Figure 6. Heterorhabditis development in Drosophila wild-type (WT) adult flies. Flies were infected with Heterorhabditis IJ (50 nematodes per fly), and incubated for 5 d at 25°C. (A) Flies were dissected using a pair of forceps to expose nematodes contained within the thorax and abdomen. (B) Pre-hermaphrodite stage of Heterorhabditis. (C) Hermaphrodite (or mother worm) stage carrying newly hatched nematodes. (D) New generation of nematodes (pre-IJ) escaping hermaphrodites into the fly body cavity. (E) Empty cuticle of Heterorhabditis hermaphrodite following the exit of the nematodes.
Future studies will use this infection protocol to test the ability of Drosophila gain-of-function or loss-of-function immune mutant flies to survive infection with axenic or symbiotic Heterorhabditis nematodes. Results from fly survival experiments combined with information on the number of nematodes that persist over-time in Drosophila immune mutants will provide important clues about the pathological events that take place in the adult fly as well as the physiological processes that determine whether the fly lives or dies following Heterorhabditis infection. As there is literally no information if and how entomopathogenic nematodes are detected by the insect immune system, further studies using this protocol together with quantitative real-time RT-PCR can be readily used to monitor the transcriptional levels of a wide range of immune recognition genes in flies at various times following infection with axenic or symbiotic worms. Similar studies could potentially lead to the identification of particular immune pathways that are activated in Drosophila adult flies following immune recognition of Heterorhabditis as well as to the number and nature of signaling genes that participate in these processes. Otherwise, WT or immune mutant flies infected with axenic or symbiotic nematodes can provide the starting material to examine the global transcription response to Heterorhabditis infection in Drosophila adults using transcriptomics.
Information on insect anti-nematode cellular responses has only recently started to emerge.49 Our nematode infection assay can be used to investigate the Drosophila cellular immune reactions (e.g., encapsulation, nodulation responses) in adult flies against Heterorhabditis, and to determine whether (and how) these reactions interact and coordinate with the humoral immune response that is also directed against the nematode parasites.34 Finally, it is possible that the current method can be applied to evaluate the interaction between host hemolymph clotting factors with the presence of Heterorhabditis in adult flies, as it has recently been shown that the clotting response plays a protective role against nematode infection in Drosophila larvae.32,33
Acknowledgments
We thank Prof Jean-Marc Reichhart (UPR9022, IBMC-CNRS, Strasbourg, France) for providing Oregon Drosophila flies and Dr David Clarke (Department of Microbiology, University College Cork, Ireland) for providing Heterorhabditis nematodes and Photorhabdus bacteria. We also thank Dr Todd Ciche (Department of Microbiology and Molecular Genetics, Michigan State University, USA) for sharing the Photorhabdus RET16 mutant strain and Prof Michael Strand for sharing the streptomycin resistant Escherichia coli strain. We finally thank members of the Biological Sciences Department at George Washington University for critical reading of the manuscript and the Columbian College of Arts and Sciences at GWU for funding.
Glossary
Abbreviations:
- IJ
infective juveniles
- Imd
immune deficiency
- PBS
phosphate-buffered saline
- WT
wild-type
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/20244
References
- 1.Vallet-Gely I, Lemaitre B, Boccard F. Bacterial strategies to overcome insect defences. Nat Rev Microbiol. 2008;6:302–13. doi: 10.1038/nrmicro1870. [DOI] [PubMed] [Google Scholar]
- 2.O’Callaghan D, Vergunst A. Non-mammalian animal models to study infectious disease: worms or fly fishing? Curr Opin Microbiol. 2010;13:79–85. doi: 10.1016/j.mib.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Limmer S, Quintin J, Hetru C, Ferrandon D. Virulence on the fly: Drosophila melanogaster as a model genetic organism to decipher host-pathogen interactions. Curr Drug Targets. 2011;12:978–99. doi: 10.2174/138945011795677818. [DOI] [PubMed] [Google Scholar]
- 4.Apidianakis Y, Rahme LG. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis Model Mech. 2011;4:21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glavis-Bloom J, Muhammed M, Mylonakis E. Of model hosts and man: using Caenorhabditis elegans, Drosophila melanogaster and Galleria mellonella as model hosts for infectious disease research. Adv Exp Med Biol. 2012;710:11–7. doi: 10.1007/978-1-4419-5638-5_2. [DOI] [PubMed] [Google Scholar]
- 6.Vlisidou I, Waterfield N, Wood W. Elucidating the in vivo targets of photorhabdus toxins in real-time using Drosophila embryos. Adv Exp Med Biol. 2012;710:49–57. doi: 10.1007/978-1-4419-5638-5_6. [DOI] [PubMed] [Google Scholar]
- 7.Schneider D. Using Drosophila as a model insect. Nat Rev Genet. 2000;1:218–26. doi: 10.1038/35042080. [DOI] [PubMed] [Google Scholar]
- 8.Charroux B, Royet J. Drosophila immune response: From systemic antimicrobial peptide production in fat body cells to local defense in the intestinal tract. Fly (Austin) 2010;4:40–7. doi: 10.4161/fly.4.1.10810. [DOI] [PubMed] [Google Scholar]
- 9.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 10.Hetru C, Hoffmann JA. NF-kappaB in the immune response of Drosophila. Cold Spring Harb Perspect Biol. 2009;1:a000232. doi: 10.1101/cshperspect.a000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganesan S, Aggarwal K, Paquette N, Silverman N. NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr Top Microbiol Immunol. 2011;349:25–60. doi: 10.1007/82_2010_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leulier F, Lemaitre B. Toll-like receptors--taking an evolutionary approach. Nat Rev Genet. 2008;9:165–78. doi: 10.1038/nrg2303. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 14.Valanne S, Wang JH, Rämet M. The Drosophila Toll signaling pathway. J Immunol. 2011;186:649–56. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- 15.Chtarbanova S, Imler JL. Microbial sensing by Toll receptors: a historical perspective. Arterioscler Thromb Vasc Biol. 2011;31:1734–8. doi: 10.1161/ATVBAHA.108.179523. [DOI] [PubMed] [Google Scholar]
- 16.Uvell H, Engström Y. A multilayered defense against infection: combinatorial control of insect immune genes. Trends Genet. 2007;23:342–9. doi: 10.1016/j.tig.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 18.Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, Swimmer C, et al. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell. 2001;1:503–14. doi: 10.1016/S1534-5807(01)00059-4. [DOI] [PubMed] [Google Scholar]
- 19.Imler JL, Bulet P. Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem Immunol Allergy. 2005;86:1–21. doi: 10.1159/000086648. [DOI] [PubMed] [Google Scholar]
- 20.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–74. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 21.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002;21:2568–79. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198:72–82. doi: 10.1111/j.0105-2896.2004.0133.x. [DOI] [PubMed] [Google Scholar]
- 23.Delaney JR, Stöven S, Uvell H, Anderson KV, Engström Y, Mlodzik M. Cooperative control of Drosophila immune responses by the JNK and NF-kappaB signaling pathways. EMBO J. 2006;25:3068–77. doi: 10.1038/sj.emboj.7601182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meister M. Blood cells of Drosophila: cell lineages and role in host defence. Curr Opin Immunol. 2004;16:10–5. doi: 10.1016/j.coi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Williams MJ. Drosophila hemopoiesis and cellular immunity. J Immunol. 2007;178:4711–6. doi: 10.4049/jimmunol.178.8.4711. [DOI] [PubMed] [Google Scholar]
- 26.Tang H. Regulation and function of the melanization reaction in Drosophila. Fly (Austin) 2009;3:105–11. doi: 10.4161/fly.3.1.7747. [DOI] [PubMed] [Google Scholar]
- 27.Eleftherianos I, Revenis C. Role and importance of phenoloxidase in insect hemostasis. J Innate Immun. 2011;3:28–33. doi: 10.1159/000321931. [DOI] [PubMed] [Google Scholar]
- 28.Davies SA, Dow JA. Modulation of epithelial innate immunity by autocrine production of nitric oxide. Gen Comp Endocrinol. 2009;162:113–21. doi: 10.1016/j.ygcen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Bae YS, Choi MK, Lee WJ. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 2010;31:278–87. doi: 10.1016/j.it.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Ryu JH, Ha EM, Lee WJ. Innate immunity and gut-microbe mutualism in Drosophila. Dev Comp Immunol. 2010;34:369–76. doi: 10.1016/j.dci.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Hallem EA, Rengarajan M, Ciche TA, Sternberg PW. Nematodes, bacteria, and flies: a tripartite model for nematode parasitism. Curr Biol. 2007;17:898–904. doi: 10.1016/j.cub.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Wilhelmsson C, Hyrsl P, Loof TG, Dobes P, Klupp M, et al. Pathogen entrapment by transglutaminase--a conserved early innate immune mechanism. PLoS Pathog. 2010;6:e1000763. doi: 10.1371/journal.ppat.1000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyrsl P, Dobes P, Wang Z, Hauling T, Wilhelmsson C, Theopold U. Clotting factors and eicosanoids protect against nematode infections. J Innate Immun. 2011;3:65–70. doi: 10.1159/000320634. [DOI] [PubMed] [Google Scholar]
- 34.Castillo JC, Reynolds SE, Eleftherianos I. Insect immune responses to nematode parasites. Trends Parasitol. 2011;27:537–47. doi: 10.1016/j.pt.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Ciche T. The biology and genome of Heterorhabditis bacteriophora. WormBook. 2007:1–9. doi: 10.1895/wormbook.1.135.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ffrench-Constant RH, Eleftherianos I, Reynolds SE. Nematode symbionts shed light on invertebrate immunity. Trends Parasitol. 2007;23:514–7. doi: 10.1016/j.pt.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Waterfield NR, Ciche T, Clarke D. Photorhabdus and a host of hosts. Annu Rev Microbiol. 2009;63:557–74. doi: 10.1146/annurev.micro.091208.073507. [DOI] [PubMed] [Google Scholar]
- 38.Ciche TA, Ensign JC. For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl Environ Microbiol. 2003;69:1890–7. doi: 10.1128/AEM.69.4.1890-1897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ffrench-Constant RH, Dowling A, Waterfield NR. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon. 2007;49:436–51. doi: 10.1016/j.toxicon.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 40.ffrench-Constant R, Waterfield N, Daborn P, Joyce S, Bennett H, Au C, et al. Photorhabdus: towards a functional genomic analysis of a symbiont and pathogen. FEMS Microbiol Rev. 2003;26:433–56. doi: 10.1111/j.1574-6976.2003.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 41.Joyce SA, Watson RJ, Clarke DJ. The regulation of pathogenicity and mutualism in Photorhabdus. Curr Opin Microbiol. 2006;9:127–32. doi: 10.1016/j.mib.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Goodrich-Blair H, Clarke DJ. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: two roads to the same destination. Mol Microbiol. 2007;64:260–8. doi: 10.1111/j.1365-2958.2007.05671.x. [DOI] [PubMed] [Google Scholar]
- 43.Clarke DJ. Photorhabdus: a model for the analysis of pathogenicity and mutualism. Cell Microbiol. 2008;10:2159–67. doi: 10.1111/j.1462-5822.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- 44.Eleftherianos I, ffrench-Constant RH, Clarke DJ, Dowling AJ, Reynolds SE. Dissecting the immune response to the entomopathogen Photorhabdus. Trends Microbiol. 2010;18:552–60. doi: 10.1016/j.tim.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Tian C, Gao B, Rodriguez MdelC, Lanz-Mendoza H, Ma B, Zhu S. Gene expression, antiparasitic activity, and functional evolution of the drosomycin family. Mol Immunol. 2008;45:3909–16. doi: 10.1016/j.molimm.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 46.Fellous S, Lazzaro BP. Larval food quality affects adult (but not larval) immune gene expression independent of effects on general condition. Mol Ecol. 2010;19:1462–8. doi: 10.1111/j.1365-294X.2010.04567.x. [DOI] [PubMed] [Google Scholar]
- 47.Fellous S, Lazzaro BP. Potential for evolutionary coupling and decoupling of larval and adult immune gene expression. Mol Ecol. 2011;20:1558–67. doi: 10.1111/j.1365-294X.2011.05006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shia AK, Glittenberg M, Thompson G, Weber AN, Reichhart JM, Ligoxygakis P. Toll-dependent antimicrobial responses in Drosophila larval fat body require Spätzle secreted by haemocytes. J Cell Sci. 2009;122:4505–15. doi: 10.1242/jcs.049155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eleftherianos I, Joyce S. ffrench-Constant RH, Clarke DJ, Reynolds SE. Probing the tri-trophic interaction between insects, nematodes and Photorhabdus. Parasitology. 2010;26:1–12. doi: 10.1017/S0031182010000508. [DOI] [PubMed] [Google Scholar]