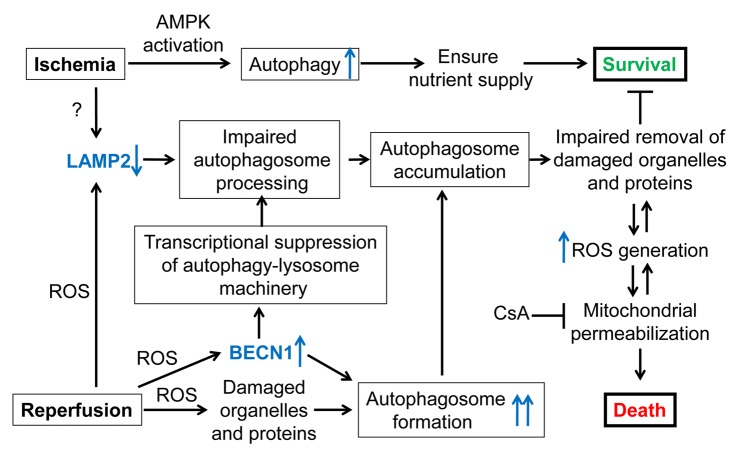
Figure 1. Schematic depicting the proposed outcomes of autophagy in myocardial ischemia-reperfusion injury. Autophagosome formation is induced by ischemia (hypoxia) via AMPK activation, and further stimulated by reperfusion (reoxygenation) secondary to ROS-induced cellular injury. Previous work suggests a critical prosurvival role for autophagy in ischemia, by ensuring nutrient supply. Our data demonstrate that both ischemia and reperfusion lead to a decline in LAMP2, which is important for autophagosome-lysosome fusion. Reperfusion-induced ROS also provokes increased BECN1 abundance, which stimulates autophagosome formation, but results in transcriptional inhibition of the autophagy-lysosome machinery. Together, these provoke marked impairment of autophagosome clearance with resultant autophagosome accumulation, and impaired removal of damaged cellular constituents. This sets up a vicious cycle of increased ROS generation and mitochondrial permeabilization, culminating in necrotic cell death, which can be attenuated by cyclosporin A (CsA), an inhibitor of mitochondrial permeability transition pore. ‘?’ indicates undefined mechanisms.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.