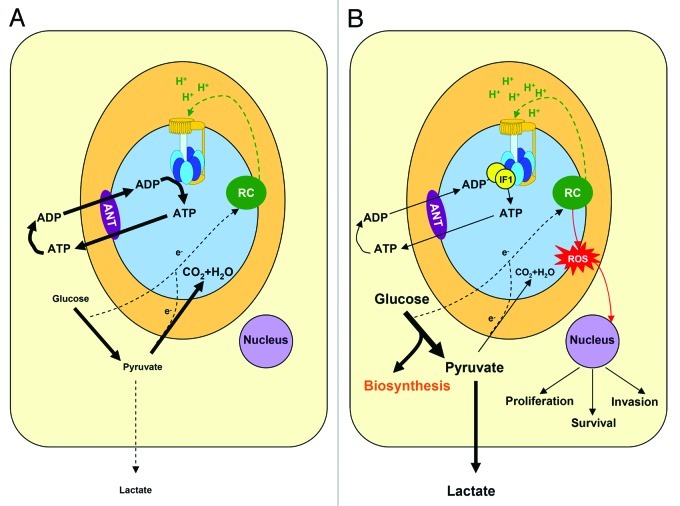
Figure 1. IF1 regulates energy metabolism in cancer cells. A, In normal aerobic cells the oxidation of glucose to CO2 and H2O is the source of electrons (e-) that feeds the respiratory chain (RC) for the generation of the proton gradient (H+, green). In oxidative phosphorylation, the H+-ATP synthase (F0, yellow rotor and F1, blue membrane protruding head) uses the proton gradient for the synthesis of ATP. The H+-ATP synthase supplies most of the ATP needed to sustain cellular activity. In the presence of oxygen the production of lactate (aerobic glycolysis) is low. B, The overexpression of IF1 in cancer cells inhibits the H+-ATP synthase and limits the flux of ATP being synthesized in oxidative phosphorylation. Consequently, aerobic glycolysis is stimulated and glucose derived carbon skeletons are diverted for biosynthetic purposes. In cancer cells, the oxidation of pyruvate in mitochondria is restrained. Inhibition of the H+-ATP synthase promotes an increase in the mitochondrial membrane potential (H+, green) and the subsequent production of superoxide radical (ROS, red). Mitochondrial ROS signal to the nucleus of the cell features of the cancer phenotype such as the promotion of proliferation, invasion and survival. The oxidation of glutamine in cancer cells is not taken into consideration for simplicity of the schematic. ANT, adenine nucleotide translocase.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.