Figure 1 .
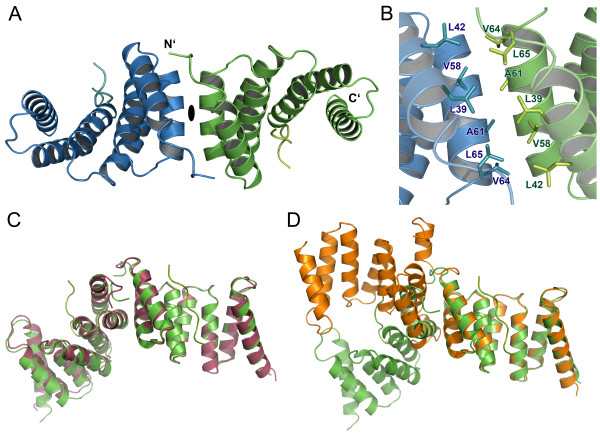
Dimer arrangement of the SycD:YopD complex. (A) Peptide-bound SycD forms a symmetric dimer, in which protomers are related by a crystallographic 2-fold axis. The dimerization is mediated via the two N-terminal helices (TPR1). (B) Dimerization interface of the SycD:YopD complex stabilized by van der Waal contacts involving the labeled residues shown as sticks. (C) Pairwise alignment (DaliLite server [29]) of SycD:YopD (green) to kinked apo SycD (red) (PDB ID: 2VGY) and to (D) monomer B of elongated apo SycD (orange) (PDB ID: 2VGX) shows that the dimer arrangement of the chaperone-peptide complex is nearly identical to the arrangement of the kinked apo form.