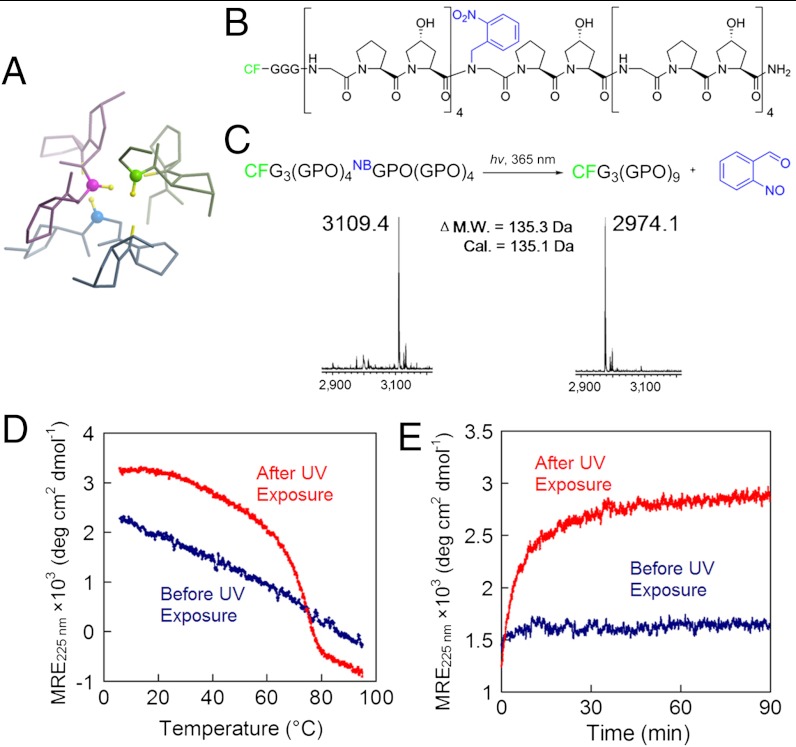
Fig. 1.
Photo-triggered triple-helical folding of caged CMPs. (A) The backbone NH groups of Gly (represented by the ball-and-stick model) play a key role in stabilizing the collagen triple helix. (B) Structure of CFNB(GPO)9 featuring the photo-reactive nitrobenzyl (NB) group (in blue) conjugated to the central Gly. (C) Photo-cleavage of the NB cage group is monitored by MALDI-MS. (D, E) The NB cage group completely abolishes the triple-helical folding capacity of CFNB(GPO)9 and the photo-cleaving of the NB cage leads to CMP folding into stable triple helices, evidenced by CD melting studies (D) and refolding kinetics (E) of the peptides before and after UV exposure. For the refolding kinetic study (E), CFNB(GPO)9 (before and after UV exposure) were thermally quenched from 80 °C to 25 °C and the change in CD ellipticity at 225 nm was monitored at 25 °C.