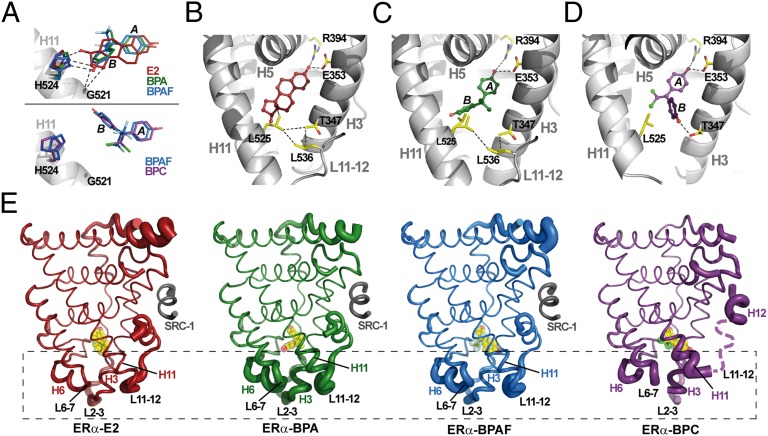
Fig. 4.
Bisphenol binding promotes ERα structural dynamics. (A) Differential interactions of bisphenols and E2 with G521 and H524. (B) The interaction of E2 with L525 strengthens a van der Waals interactions network involving T347 (H3), L525 (H11), and L536 (L11–L12). (C) Because of a lack of contact with BPA, L525 is not stabilized and adopts two different conformations. (D) In the BPC-bound ERα structure, T347 rotates by 180° to form a hydrogen bond with the bisphenol, resulting in the disruption of the hydrophobic network. (E) Ribbon representation of ERα LBD in complex with E2 (red), BPA (green), BPAF (blue), and BPC (purple; dashed line denotes missing residues). Ligands are shown in yellow. The diameter of the ribbon is directly proportional to the temperature factor B and highlights the dynamics all along the polypeptide chain.