Fig. 3.
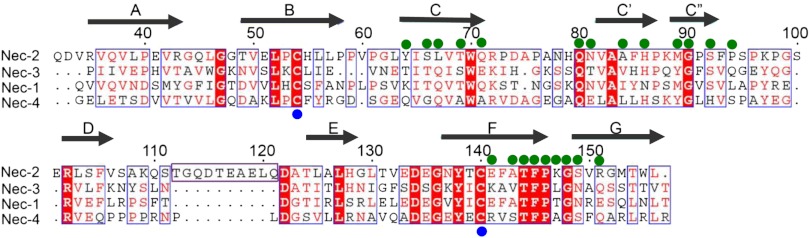
Structure-based sequence alignment of human nectins. The secondary structure of nectin-2 IgV domain is labeled on the top of the alignment. Residues with similar properties are marked in red, whereas identical residues are in white with red shading. Interfacial residues (green circle) and the cysteine residues involved in the formation of disulfide bond between B and F strands (blue circle) are marked. The long loop between the D and E strands of nectin-2, which is absent in other nectins, is also shown.
