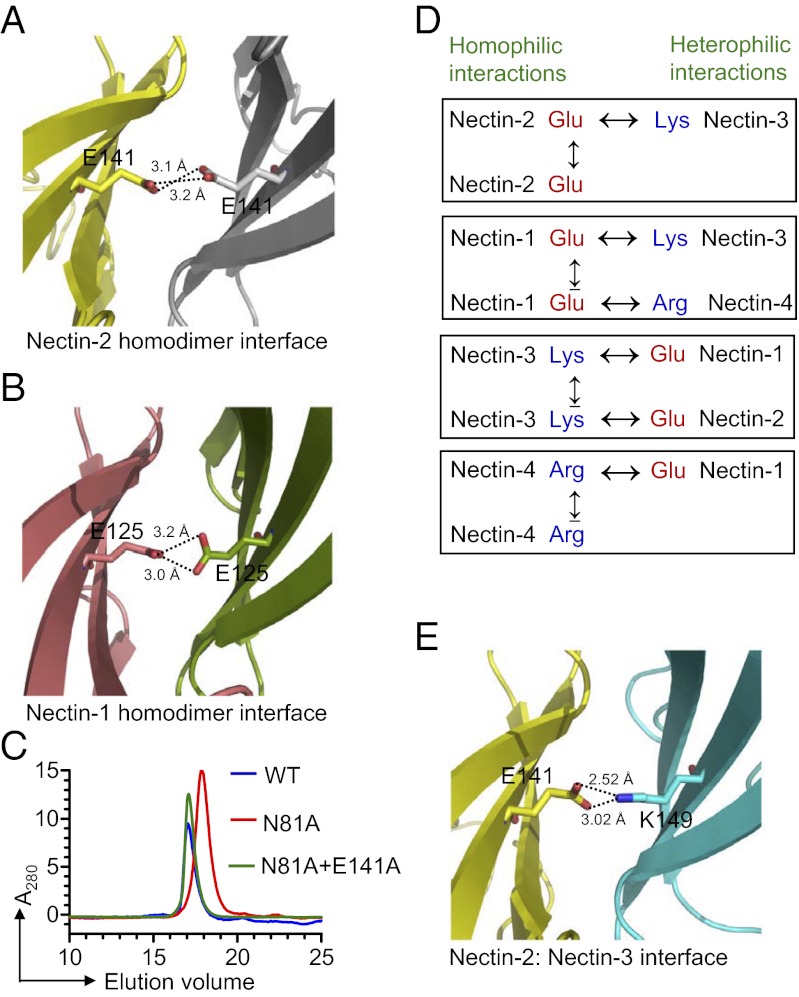
Fig. 4.
(A) Dimer interface of human nectin-2 showing the close proximity of two negatively charged side chains; Glu-141 is contributed from the F strand of each monomer (yellow and gray). (B) Dimeric interface of nectin-1 (PDB ID code 3ALP) showing the similar unfavorable repulsive electrostatics as depicted in the case of nectin-2. (C) Size-exclusion chromatography showing that the E141A mutation in the background of the N81A mutation causes the shift of monomer (an effect of N81A mutation) to the dimer population. (D) The presence of the same charges at the homodimer interface causes unfavorable electrostatics during homophilic interactions, whereas the presence of two different charges at the heterodimer interface can eliminate the unfavorable electrostatics as seen at the homodimer interface. (E) Molecular model showing the heterophilic interaction between nectin-2 (yellow) and nectin-3 (cyan). The modeling suggests that E141 of nectin-2 contacts K149 of nectin-3, forming a putative H-bond at the center of the interface, which favors a strong heterophilic interaction.