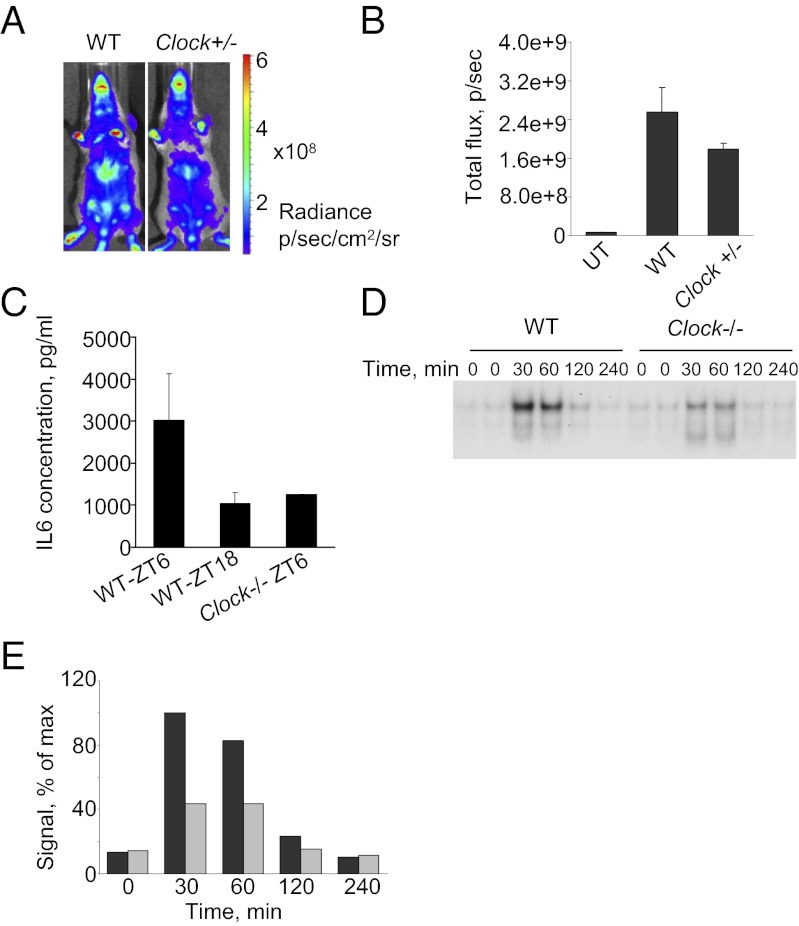
Fig. 5.
NF-κB activation in response to CBLB502 is reduced in liver and primary hepatocytes of Clock-deficient mice. (A) BALB/C-Tg(IκBα-Luc) mice were crossed to C57BL/6J WT or Clock-deficient mice (C57BL/6J background). The progeny (WT or Clock+/−) received 1 ug of CBLB502 at ZT06 and were monitored for luciferase expression in vivo 2 h later. Representative images for each genotype are shown. (B) Quantitative analysis of luciferase expression in livers of WT (n = 3) and Clock+/− (n = 3) mice measured 2 h post CBLB502 administration. CBLB502-mediated NF-κB activation is reduced in Clock+/− mice (P < 0.05). UT, untreated (C) CBLB502-mediated induction of IL-6 correlates with the scale of NF-κB activation. Two WT and two Clock−/− mice received a single injection of CBLB502 at the times indicated. Blood was collected 2 h later, and the plasma concentration of IL-6 was measured by ELISA. (D) Impaired induction of NF-κB DNA binding in primary hepatocytes of Clock−/− mice treated with CBLB502 in vitro. Primary hepatocytes isolated from age-matched WT and Clock−/− mice were treated with 100 ng/mL of CBLB502 for the indicated times. (E) Quantitative analysis of the intensity of the band corresponding to DNA-bound p65/p50 dimer. The amount of DNA-bound complex in Clock-deficient hepatocytes (gray bars) is reduced significantly compared with WT hepatocytes (black bars).