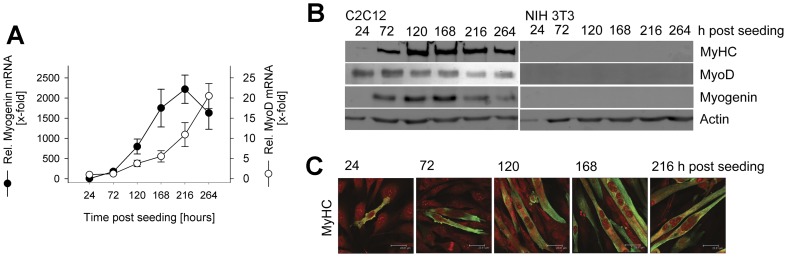
Figure 1. In vitro differentiation of mouse-derived C2C12 SkMCs.
C2C12 myoblasts were cultivated in differentiation medium from 24 hours after seeding onwards for different periods of time as indicated. NIH/3T3 fibroblasts were cultivated in parallel and were used as controls. (A) Total RNA was isolated, and after reverse transcription of mRNA, cDNA was amplified by quantitative real-time PCR. The relative abundance of myogenin (filled symbols) and MyoD (open symbols) mRNA was normalized to that of β-actin mRNA, and was compared to the mRNA level before differentiation, i.e. at 24 hours post seeding. Data represents means ± S.E.M. from three independent experiments. (B) Complete cellular protein extracts were separated by SDS-PAGE, transferred to nitrocellulose, and labeled with antibodies recognizing muscle cell-specific myosin heavy chain (MyHC), MyoD, myogenin, or β-actin as loading control. Immune complexes were visualized with HRPO-conjugated secondary antibodies and enhanced chemiluminescence. Results from a representative experiment out of three are depicted. (C) After fixation, cells were immunolabeled using anti-MyHC primary antibody and Cy2-conjugated secondary antibody (green fluorescence), and were counterstained using propidium iodide (red fluorescence). Representative images were obtained by confocal laser scanning microscopy.