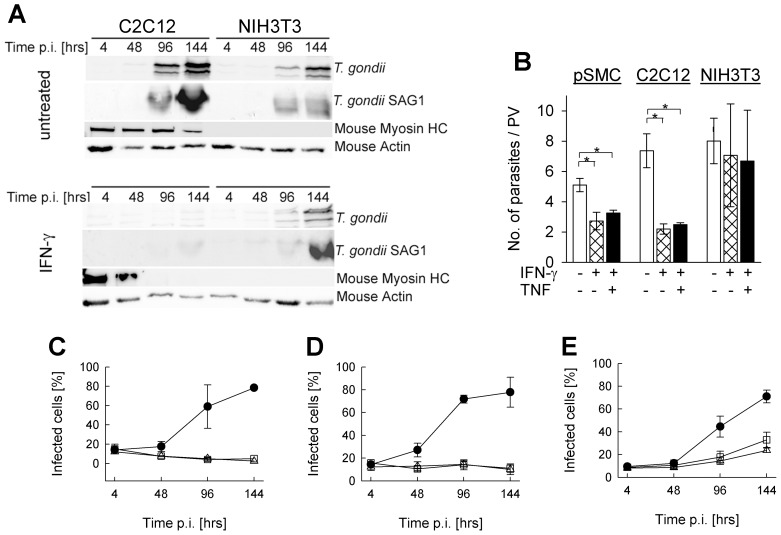
Figure 3. Impact of IFN-γ and TNF on Toxoplasma development in SkMCs.
Primary embryonic SkMCs or C2C12 SkMCs were differentiated to mature myotubes. They were, together with control NIH/3T3 fibroblasts, infected with T. gondii NTE tachyzoites (parasite-host cell ratio 2∶1). At the time of infection, cells were stimulated or not with 100 U/ml IFN-γ alone or combined with 100 pg/ml TNF. (A) At different time points post infection (p.i.), C2C12 and NIH/3T3 cells were lysed, and soluble proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were probed with anti-T. gondii serum, anti-T. gondii SAG1, anti-mouse myosin HC, or anti-mouse actin and appropriate HRPO-conjugated secondary antibodies. Immune complexes were visualized by enhanced chemiluminescence. (B) Forty-eight hours after infection, cells were fixed and permeabilized, and T. gondii was visualized by immunofluorescence staining. The total cell population was stained with propidium iodide. The number of parasites was counted in at least 100 parasitophorous vacuoles (PV) per sample. Data represents the mean number of parasites per PV ± S.E.M. from at least 3 independent experiments; significant differences between non-stimulated and stimulated cells are indicated (*p<0.05). (C–E) At the time of infection, primary SkMCs (C), C2C12 (D) or NIH/3T3 (E) cells were stimulated with IFN-γ alone (open squares), IFN-γ combined with TNF (open triangles), or were left untreated (closed symbols). Cells were fixed at different time points after infection, and T. gondii and total cells were visualized by immunolabelling and propidium iodide staining, respectively. After inspection of at least 500 cells per sample, the percentages of infected cells were calculated. Results are means ± S.E.M. from at least 3 independent experiments.