Abstract
Urinary excretion of albumin (UAlb) is used clinically as a marker of diabetic nephropathy (DN). Although DN was thought to be a unidirectional process, recent studies demonstrated that a large proportion of patients diagnosed with DN reverted to normoalbuminuria. Moreover, despite the normoalbuminuria, one-third of them exhibited reduced renal function even during the microalbuminuric stage. This study was performed to investigate whether urinary angiotensinogen (UAGT) level may serve as a useful marker of the early stage of experimental type 1 diabetes (T1DM). T1DM was induced by a single intraperitoneal injection of streptozotocin. Control mice were injected with citrate buffer. Two days after streptozotocin injection, half of the mice received continuous insulin treatment. Our data showed that UAlb excretion was increased 6 days after streptozotocin injection compared to controls, whereas UAGT excretion was increased at an earlier time point. These increases were reversed by insulin treatment. The UAGT to UAlb ratio was increased in diabetic mice compared to control mice. Furthermore, the increased AGT expression in the kidneys was observed in diabetic mice. These data suggest that UAGT might be useful as a novel early biomarker of activation of the renin–angiotensin system in experimental type 1 diabetes.
Keywords: streptozotocin-induced type 1 diabetic mice, angiotensinogen, biomarker, renin–angiotensin system, diabetic nephropathy
Introduction
Microalbuminuria is the most commonly used early clinical indicator of diabetic nephropathy (DN) in both type 1 and type 2 diabetic patients (1, 2). Previously, DN was thought to be a unidirectional process that starts with microalbuminuria and leads to end-stage renal failure (3). Therefore, the presence of albumin (Alb) in the urine has been considered predictive of the subsequent development and clinical progression of DN. However, it was recently shown that a large proportion of type 1 diabetic patients with DN revert to normoalbuminuria, of which one-third of them exhibited reduced renal function even during the microalbuminuric stage (4). Therefore, a more sensitive and specific marker for DN, rather than urinary Alb (UAlb) excretion, is needed. The mechanisms of DN pathogenesis are multifactorial (5, 6). In 1985, a clinical trial indicated that the activation of the intrarenal renin–angiotensin system (RAS) had a potential role in the mechanism of DN (7). Since then, it has been reported that angiotensin-converting enzyme (ACE) inhibitors, angiotensin II-receptor blockers (ARBs), or a dual blockade of the RAS (ACE inhibitors and ARBs) provide renoprotection in patients with type 1 (8, 9) or type 2 (10 – 14) diabetes, and some reports show the result is independent of systemic blood pressure changes (10, 13).
Others and we have previously reported that the intrarenal RAS plays an important role in the progression of DN (15 – 20). Especially, angiotensinogen (AGT) expression in the kidneys is consistently increased in the diabetic condition. Moreover, urinary AGT (UAGT) level was enhanced in Zucker diabetic fatty type 2 diabetic rats (21) and db/db type 2 diabetic mice (22). Recent evidence suggests that AGT is constitutively released not only in the liver but also in the proximal tubules of the kidney (6). AGT is the only known substrate for renin, which is the rate-limiting enzyme of the RAS (23, 24). Because the concentration of AGT is close to the Michaelis-Menten constant for renin, not only renin levels but also AGT levels are thought to affect the formation rate of angiotensin peptides (25, 26).
Recently, clinical studies showed that UAGT level could be a potential marker of the intrarenal RAS status in diabetes (27, 28). In hypertensive DN patients who received ARBs, urinary levels of AGT, Alb, inflammatory markers, and oxidative stress, as well as blood pressure were decreased. This suggests that the renoprotective effect of ARBs may involve suppression of intrarenal AGT levels in the patients with type 2 diabetes (27). Furthermore, an increase in UAGT levels was higher in normoalbuminuric patients with type 1 diabetes compared to control subjects, even though increased AGT was not observed in plasma (28). These observations suggest that UAGT may function as a candidate early marker for intrarenal RAS activation. To confirm our hypothesis, we investigated UAGT levels using a streptozotocin (STZ)-induced type 1 diabetic mouse model with clear onset of diabetes. This model does not have complications related to obesity and hyperlipidemia that are also associated with RAS activation; therefore, we can investigate the role of AGT after the induction of hyperglycemia, demonstrating the importance of UAGT level in patients with type 1 diabetes.
In this study, we tested the hypothesis that increased UAGT excretion is present prior to the onset of UAlb in STZ-induced type 1 diabetic mice, and UAGT might be useful as an early biomarker of activation of the RAS in experimental type 1 diabetes.
Materials and Methods
Research design
All procedures and protocols used in this study were approved by the Institutional Animal Care and Use Committee of the Tulane University Health Sciences Center. Animals used in this study were male Hsd:ICR mice (CD-1®; Harlan Laboratories, Indianapolis, IN, USA). Mice were maintained in 12-h light/dark cycles with access to food and water ad libitum (29). At 6 weeks of age, CD-1 mice received a single intraperitoneal injection of 200 mg/kg STZ (Fisher Health Care, Houston, TX, USA) dissolved in 0.05 M citrate buffer, pH 4.5 (n = 10). Citrate buffer alone was used in control mice (n = 10, day 0) (29, 30). Two days after STZ injection (day +2), half of the mice (n = 10) received sustained-release insulin implants (LinBit®; insulin release rate: approximately 0.1 unit/day per pellet for over 30 days, 1 pellet per mouse) (LinShin Canada, Inc., Scarborough, Ontario, Canada) (31, 32). Body weight and postprandial blood glucose (Glucometer, One Touch Ultra®; LifeScan Inc., Milpitas, CA, USA) were monitored 1 day prior to STZ injection (Before Treatment) and daily thereafter. Twenty-four-hour urine samples were collected on day −2 (Before Treatment), day +3, and day +6 using metabolic cages (Hatteras Instruments, Southbank Drive Cary, NC, USA). Water consumption was measured on day −2 (Before Treatment), day +3, and day +6. Seven days after STZ injection (day +7), all mice were sacrificed and kidney samples were harvested.
Enzyme-linked immunosorbent (ELISA) assays
Urinary neutrophil gelatinase–associated lipocalin (UNGAL), Alb, and AGT analyses were performed with ELISA kits according to the manufacturer’s instructions. Using 24-h urine samples, the UNGAL concentration was examined by a mouse NGAL ELISA kit® (Cedarlane, Burlington, NC, USA) (33). UAlb excretion and UAGT excretion were measured using mouse albumin ELISA® (Immunology Consultants Laboratory, Inc., Newberg, OR, USA) (34) and the mouse total angiotensinogen assay kit® (Immuno-Biological Laboratories Co., Ltd., Takasaki) (21), respectively. The ratio of UAGT to UAlb (UAGT/UAlb) was calculated using each data set (ng·µg−1). Using urinary excretion levels of Alb, AGT, and UAGT/UAlb ratio, we calculated the %change in UAlb, %change in UAGT, and %change in UAGT/UAlb using the following formula:
Urinary protein (UPro) assay
UPro excretion over a 24-h period was evaluated using the Bradford protein assay (Bio-Rad, Hercules, CA, USA) (35). As a standard, rat serum protein (Chondrex, Redmond, WA, USA) was used.
Quantitative real-time RT-PCR
RNAs were extracted from the tissues using the RNeasy Mini kit® (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions. Expression of the AGT gene in the kidney was examined using the Brilliant II QRT-PCR Master Mix kit, 1-Step® (Stratagene, Santa Clara, CA, USA) following the RTPCR conditions according to the manufacturer’s instructions. Quantitative real-time RT-PCR was performed as previously described (36, 37). Data from quantitative real-time RT-PCR were normalized to β-actin mRNA expression. Primer sequences were as follows: AGT, forward primer 5′-TAT CCA CTG ACC CAG TTC TT-3′, reverse primer 5′-AAG TGA ACG TAG GTG TTG AAA-3′, probe 5′-/6-FAM/CTG TGA CAG GGT GGA AGA TGA ACT TGC CA/3BHQ-1/3′; β-actin, forward primer 5′-ATC ATG AAG TGT GAC GTT GA-3′, reverse primer 5′-GAT CTT CAT GGT GCT AGG AGC-3′, probe 5′/-6-FAM/TCT ATG CCA ACA CAG TGC TGT CTG GT/3BHQ-2/3′.
Immunohistochemical (IHC) staining
The level of expression of AGT protein in the kidneys was examined by IHC as described previously (37, 38). Renal sections were fixed in 4% paraformaldehyde, embedded in paraffin, and deparaffinized in xylene. Then, 3-µm-thick tissue sections were prepared by an out-sourcing company (Mass Histology Service, Worcester, MA, USA). IHC was performed with an automated system (Autostainer; Dako, Carpinteria, CA, USA) and slides were counterstained with hematoxylin. The primary antibody against rat polyclonal AGT was obtained from Immuno-Biological Laboratories Co., Ltd. Semi-quantitative analysis of the level of immunoreactivity was evaluated in a blind manner using a semi-automatic image analysis system that employed the Image-Pro plus software (Media Cybernetics, Bethesda, MD, USA) as previously described (37 – 40).
Statistical analyses
All data are presented as the means ± S.E.M. Data of the animal profiles, urine samples, and AGT expression were evaluated by the one-way repeated-measures ANOVA or one-way factorial ANOVA with Dunnett’s test as a post-hoc test. P < 0.05 was considered to be statistically significant. All of the computations, including data management and statistical analyses, were performed with JMP software (SAS Institute, Cary, NC, USA).
Results
Animal profiles
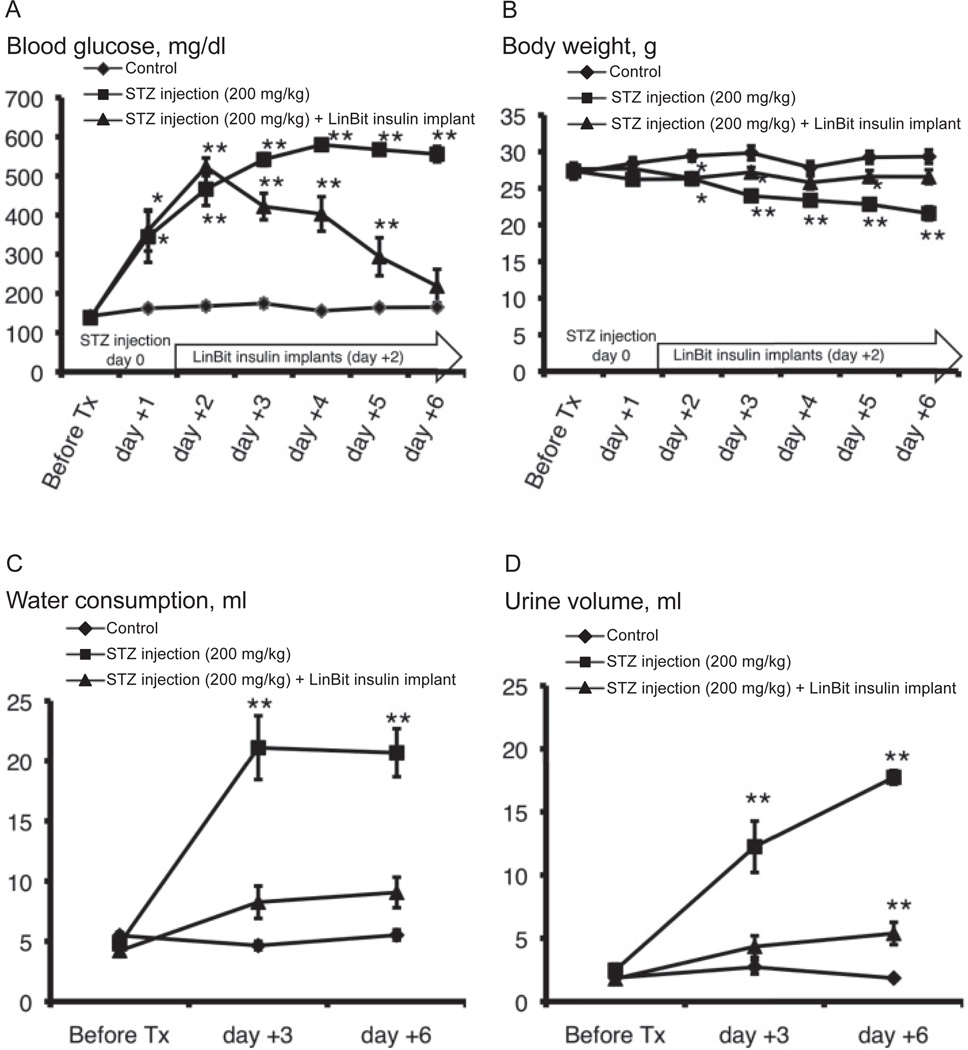
The postprandial blood glucose levels and body weights of the mice are shown in Fig. 1. The postprandial blood glucose levels were elevated 1 day after STZ injection with respect to those in the control group. Two days after STZ injection, all mice were diabetic (> 400 mg/dl) (30). The change in postprandial blood glucose was relieved by insulin treatment during the experiment (Fig. 1A). Body weights of the diabetic mice were significantly lower than those of the control mice. Insulin treatment offset this change (Fig. 1B).
Fig. 1.
Changes in postprandial blood glucose (A), body weight (B), water consumption (C), and urine volume (D) in control, STZ-induced type 1 diabetic, and LinBit insulin–treated diabetic mice before and after a single intraperitoneal injection of STZ. Data are expressed as the mean ± S.E.M. *P < 0.05, **P < 0.01 vs. control mice. STZ, streptozotocin.
Water consumption and urine volume
Water consumption by mice of the STZ group was markedly greater than that of the control group 3 and 6 days after STZ injection (P < 0.01 vs. control). This effect was relieved by insulin treatment (Fig. 1C). The 24-h urine volume in mice of the STZ group was markedly greater than that of the control group 3 and 6 days after STZ injection (P < 0.01 vs. control). The change in urine volume was mitigated by insulin treatment (Fig. 1D).
Measurement of UNGAL concentration and temporal profiles of UAlb, UAGT, and UPro excretions
It is known that STZ occasionally induces acute kidney injury (AKI). Before the assays using urine samples, the UNGAL concentration (ng/ml) was measured, as it may be used as an early biomarker of AKI (cut-off line: UNGAL concentration higher than 200 ng/ml) (41). As a result, all mice used in the assays were precluded of AKI (urine samples on day +6: control group, 35 ± 7 ng/ml; STZ injection group, 24 ± 4 ng/ml; STZ injection + LinBit insulin implant group, 46 ± 12 ng/ml).
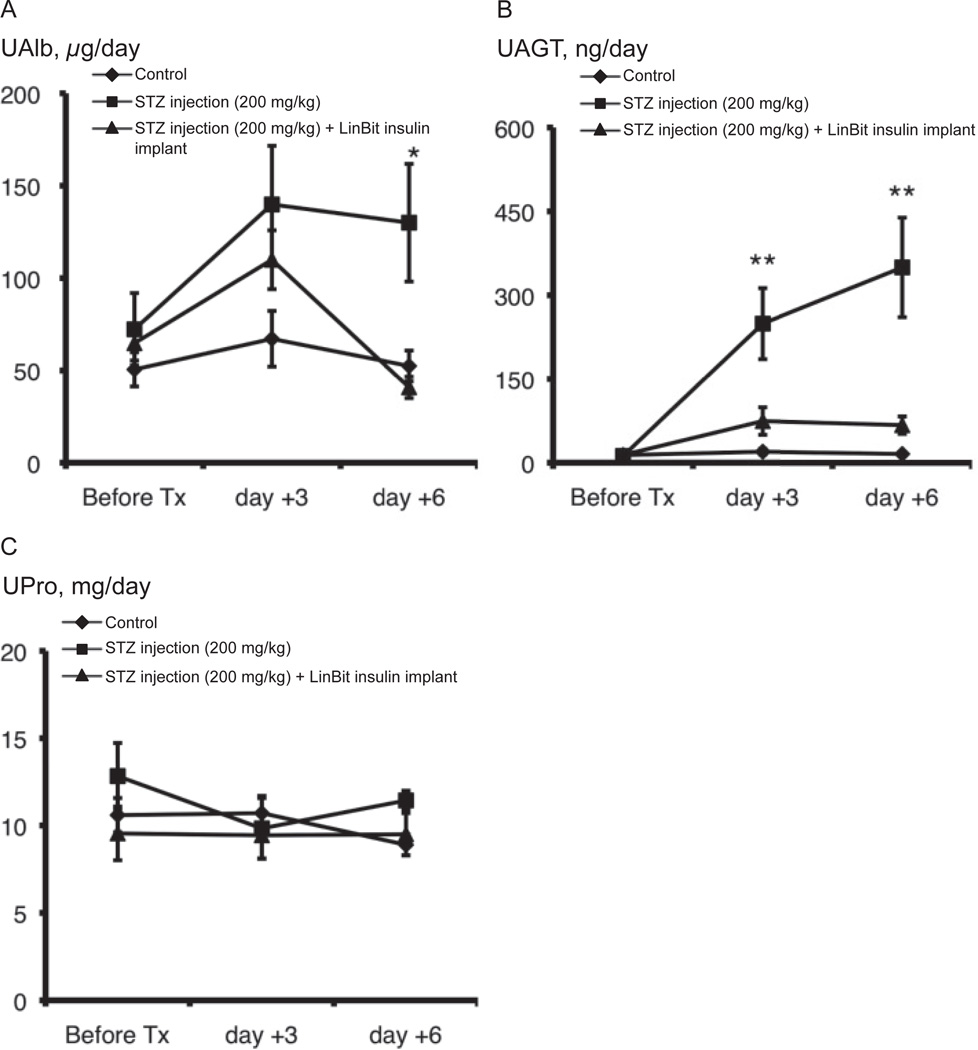
Using urine samples from mice that were non-exhibitive of AKI, UAlb, UAGT, and UPro excretion were measured. Before STZ injection and on day +3 after STZ injection, UAlb excretion was not different among the 3 groups. However, UAlb excretion increased 6 days after STZ injection (130.0 ± 31.8 µg/day) with respect to the controls (52.5 ± 8.4 µg/day) (P = 0.0159). This change was completely negated by insulin treatment (40.8 ± 5.8 µg/day) (Fig. 2A). UAGT excretion was higher in the STZ group (249.2 ± 63.5 µg/day) than in the control group (19.7 ± 4.9 µg/day) (P = 0.0006) 3 days after STZ injection, and it continued to increase in the STZ group (349.6 ± 89.1 µg/day) while the control group (15.9 ± 2.2 µg/day) stayed constant 6 days after STZ injection (P = 0.0002). This change was also completely negated by insulin treatment (on day +3: 74.5 ± 24.6 µg/day, on day +6: 67.3 ± 15.5 µg/day) (Fig. 2B). UPro excretion was not significant in all groups (Fig. 2C).
Fig. 2.
Changes in UAlb (A), UAGT (B), and UPro (C) excretion in control, STZ-induced diabetic, and LinBit insulin–treated diabetic mice. Urine samples were collected before STZ injection and on days +3 and +6 after STZ injection. Data are expressed as the mean ± S.E.M. *P < 0.05, **P < 0.01 vs. control mice. UAlb, urinary albumin; UAGT, urinary angiotensinogen; UPro, urinary protein; STZ, streptozotocin.
Ratio of UAGT/UAlb and the %change in UAlb, UAGT, and UAGT/UAlb
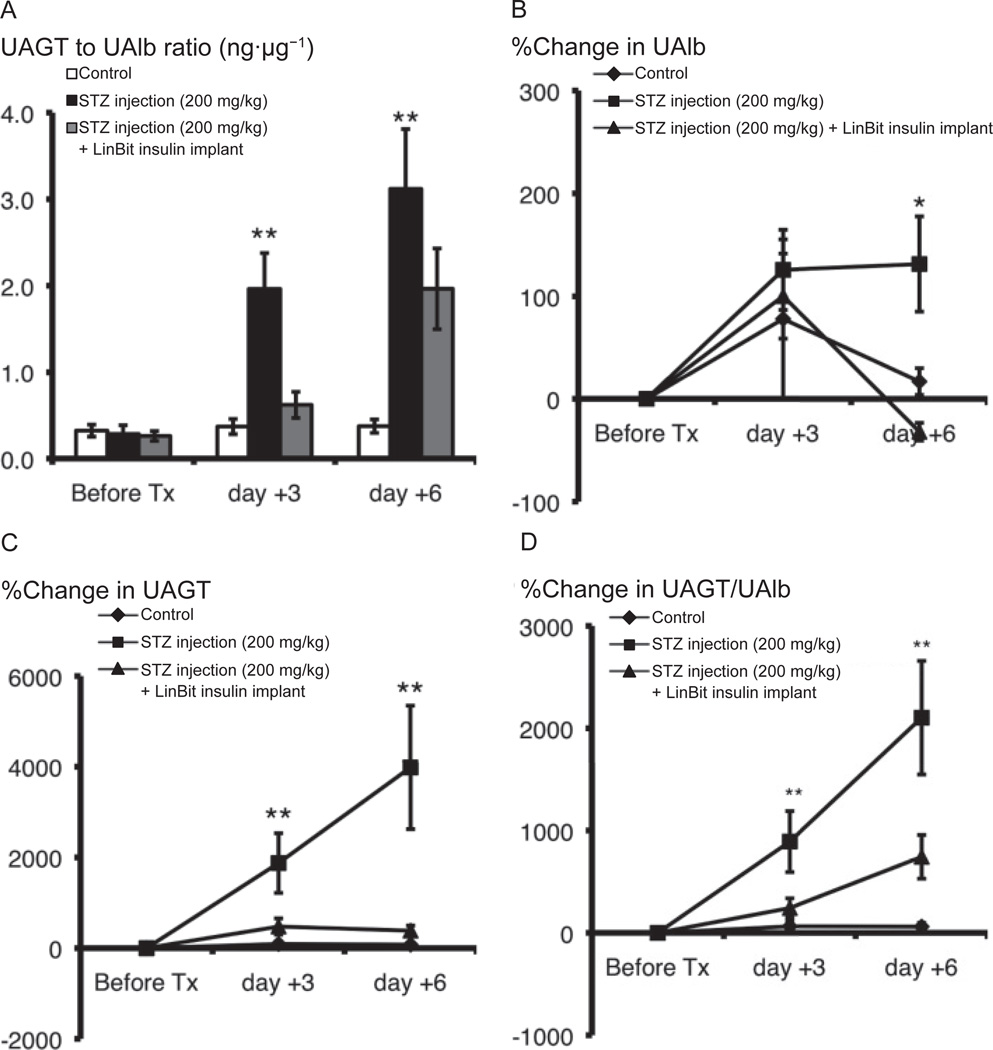
The UAGT/UAlb ratio (ng·µg−1) was statistically higher in the STZ group than in the control group 3 and 6 days after STZ injections (P < 0.01) (Fig. 3A). This effect was completely mitigated by insulin treatment. Although the %change in UAlb did not differ significantly between the control and STZ groups 3 days after STZ injection, the %change in UAlb increased 6 days after STZ injection (131.2% ± 46.3%) with respect to the controls (16.9% ± 13.0%) (P = 0.0105). This change was completely abolished by insulin treatment (−31.9% ± 8.6%) (Fig. 3B). The %change in UAGT was higher in the STZ group (1875.7% ± 657.7%) than in the control group (91.2% ± 53.9%) (P = 0.0044) 3 days after STZ injection and continued to increase in the STZ group (3985.9% ± 1362.4%) with respect to the control group (76.5% ± 41.6%) (P < 0.00001) 6 days after STZ injection. This change was also completely abolished by insulin treatment (on day +3: 476.5% ± 179.0%, on day +6: 385.1% ± 112.9%) (Fig. 3C). The %change in UAGT/UAlb ratio was higher in the STZ group (892.2% ± 297.9%) than in the control group (64.1% ± 52.9%) (P = 0.0046) 3 days after STZ injection and continued to increase in the STZ group (2103.0% ± 555.0%) with respect to the control group (60.7% ± 37.8%) 6 days after STZ injection (P = 0.0007). This change was also completely abolished by insulin treatment (on day +3: 242.8% ± 95.7%, on day +6: 742.7% ± 213.9%) (Fig. 3D).
Fig. 3.
Ratio of UAGT/UAlb (ng·µg−1) (A) and %change in UAlb (B), UAGT (C), and UAGT/UAlb (D) in control, STZ-induced diabetic, and LinBit insulin–treated diabetic mice were calculated using urinary excretion data. Data are expressed as the mean ± S.E.M. *P < 0.05, **P < 0.01 vs. control mice. UAGT, urinary angiotensinogen; UAlb, urinary albumin; STZ, streptozotocin.
AGT mRNA expression in the kidneys of STZ-induced diabetes with or without insulin treatment
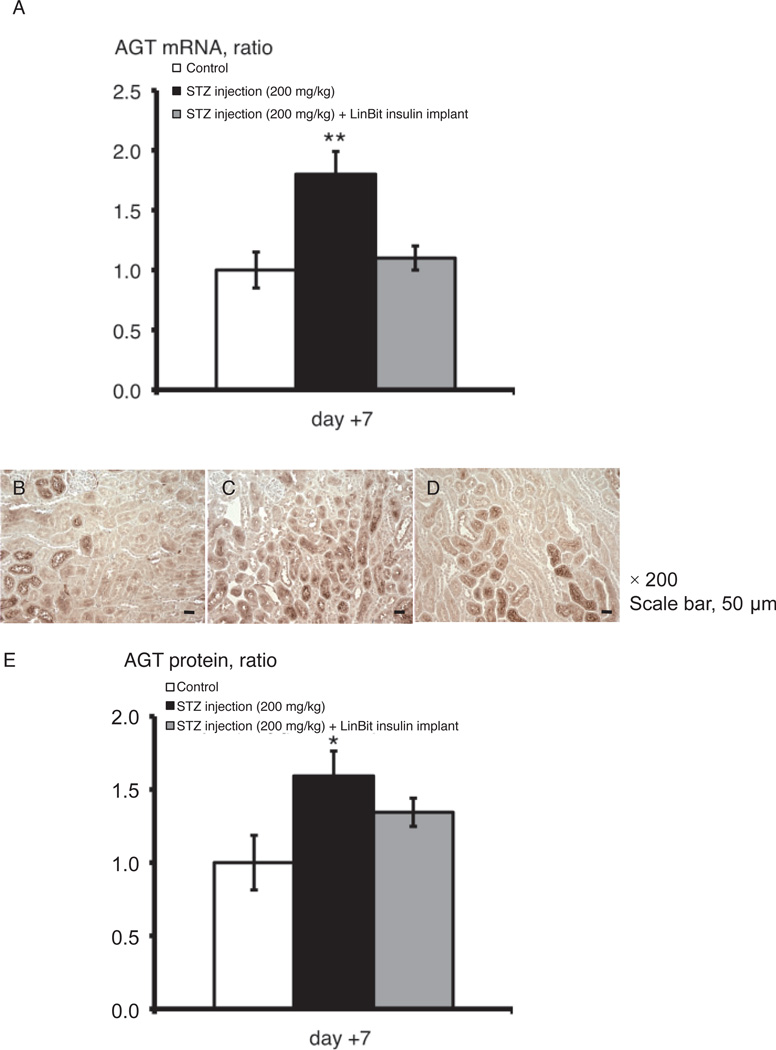
Kidney samples harvested 7 days after STZ injection were used to detect AGT mRNA levels. Increased AGT mRNA expression in the kidneys was observed in diabetic mice (P < 0.01), and this increase was negated with insulin treatment (Fig. 4A).
Fig. 4.
AGT mRNA expression (A) and immunohistochemical localization of AGT protein (B, C, D, and E) in the kidneys of control, STZ-induced diabetic, and LinBit insulin–treated diabetic mice. We used kidneys on day +7 after STZ injection. Immunohistochemical staining (magnification, × 200; scale bar, 50 µm) shows increased AGT in the kidneys of diabetic mice (C) compared with control (B) or LinBit insulin–treated diabetic mice (D). AGT protein expression levels are indicated in panel E. Data are expressed as the mean ± S.E.M. *P < 0.05, **P < 0.01 vs. control mice. AGT, angiotensinogen; STZ, streptozotocin.
AGT protein expression in the kidneys of STZ-induced diabetes with or without normalization with insulin treatment
Kidney samples harvested 7 days after STZ injection were used to detect AGT protein levels. IHC staining for AGT protein (Fig. 4: B, C, and D) was clearly of greater intensity in the kidneys (especially in the proximal tubules) of diabetic mice than in those of control mice (P < 0.05), and this effect was mitigated by insulin treatment (Fig. 4E).
Discussion
In this study we demonstrate that UAGT and UAlb excretion is elevated in STZ-treated T1DM mice. The following novel findings emerged: UAGT excretion accompanied by the intrarenal AGT expression increase earlier than UAlb after STZ injection compared to controls. Moreover, the UAGT to UAlb ratio is increased in diabetic mice compared to control mice. Our data revealed conclusive evidence that UAGT is an early biomarker of increased RAS in T1DM.
In our study we have used STZ to induce T1DM. STZ is widely used to induce type 1 diabetes in rodent models (30, 42, 43). However, it is known that in some cases STZ can induce AKI. STZ is an unusual aminoglycoside containing a nitrosoamino group, which can cause acute toxic injury to the kidney (44). Therefore, before the assays using urine samples, the UNGAL concentration was measured to detect AKI. NGAL concentration is usually used as an early biomarker of AKI (41, 45). The mice used in this study did not exhibit AKI, only exhibiting the expected signs of STZ-induced type 1 diabetes.
UAGT excretion is higher in patients with type 1 diabetes compared to control subjects (28). Despite the importance of the RAS in the development of DN, the significance of intrarenal RAS, especially the role of AGT, in the early stage of T1DM is not yet fully elucidated. Here, we investigated UAGT level as a candidate marker of activation of the RAS in type 1 diabetes. STZ-induced type 1 diabetic mice showed typical symptoms of diabetes mellitus and insulin treatment ameliorated these changes in agreement with short-term investigation studies (46, 47). After development of type 1 diabetes, we observed increases in excretion levels of UAlb and UAGT in diabetic mice compared to control and insulin-treated groups. These observations indicate that urinary excretions of AGT and Alb were increased in the STZ-treated mice compared to the control animals. An important observation is that the augmented excretion level of AGT in the urine was apparent before the development of increased Alb levels. This could be because the UAGT level is highly sensitive to the onset of nephropathy. Furthermore, our data showed that the ratio of UAGT to UAlb was significantly higher in the STZ group than in the control group. Because insulin treatment mitigated the increased excretion of UAlb and UAGT, it is thought that increased glucose level induced elevated UAlb and UAGT excretions. Although we have measured a late-appearing marker of kidney injury, UPro excretion, we did not find any significant difference between groups. Our results indicate that augmented UAGT level might potentially serve as a novel, early biomarker of intrarenal RAS activation in experimental type 1 diabetes. These findings could lead to earlier diagnosis of diabetic complications and start of medical treatment. They also suggest that we should re-assess the clinical significance of RAS blockade.
In the investigation of biomarker using urinary samples, the suspicion that enhanced UAGT excretion in a diabetic model is just a non-specific consequence of damaged glomerular filtration barrier remains. To address this issue, we monitored AGT expression in the kidneys. AGT mRNA and protein expression in the kidneys, especially in the proximal tubules, were increased in the STZ group compared to the control group. This finding is congruent with previous reports demonstrating that intrarenal AGT is formed primarily in the proximal tubule cells and is enhanced in diabetic conditions (17, 18, 21, 48, 49). Although the detailed localization of AGT mRNA and protein in the convoluted and straight proximal tubules has not been yet clarified (50 – 54), our recent data suggest that AGT mRNA in the straight tubules and AGT protein in the convoluted and straight proximal tubules are important and need to be investigated in future studies (55). The data in the kidneys may rule out the possibility of a damaged glomerular filtration barrier. Because these increased mRNA and protein levels were ameliorated in the insulin-treated group, we suggest that increased blood glucose induces intrarenal AGT mRNA and protein expression and a subsequent increase in UAGT excretion.
The pathogenesis of DN is clearly multifactorial (5, 6, 56, 57). The activation of the intrarenal RAS has been shown to have a potential role in the mechanism of DN (6, 19, 29, 58 – 60), as shown in large-scale clinical trials (8 – 13). Recently, the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study (13) provided novel insights that olmesartan, an ARB, was associated with delayed onset of microalbuminuria, even though blood pressure control in the patients with type 2 diabetes was excellent. This result suggests the possibility of preemptive treatment of ARB for the delay or prevention of microalbuminuria in diabetes (14). Based on the outcome of this large-scale clinical trial, we expect the intrarenal AGT to have an important role in the development of DN because we already reported that ARB treatment suppressed intrarenal and urinary AGT in animal studies (16, 61) as well as in clinical studies (27, 62).
Various proteins, such as urinary type IV collagen, adiponectin, and advanced glycation end products, have been implicated as potential markers of DN (63 – 65). However, such divergent findings have complicated the search for a reliable biomarker. AGT is the only known substrate for renin, which is the rate-limiting enzyme of the RAS (23, 24). Upregulation of AGT levels may lead to elevation of the angiotensin peptide levels (25, 26). Our recent studies in diabetic models have documented the involvement of AGT in activation of the RAS (16, 27, 28). While intrarenal renin expression is also increased in diabetic animals (16), some in vitro reports (66 – 72) supported that high glucose augmented AGT gene expression using a cell line of rat immortalized renal proximal tubular cells. Using the same cell line, it was also reported that the insulin treatment inhibited the stimulatory effect of high glucose on the expression of AGT. Thus, AGT plays an important role in the mechanism of high glucose–induced AGT expression and the development of diabetic complications including nephropathy.
A shortcoming of this study is the exclusion of a long-term study for the role of AGT in the development and progression of DN. However, it was repeatedly shown that STZ-induced T1DM leads to DN in long-term studies (29 – 32). Here, we focused on the initial increase of UAGT in a short-term study and showed that increased UAGT may signal the development of RAS. Moreover, we have reported on the augmented AGT expression and RAS activation in the progression of DN (16 – 18). Based on our previous data and the results of this study, we are projecting future clinical studies to focus on the role and mechanisms of AGT in the onset of nephropathy and the progression of kidney dysfunction, as well as their relationships.
Acknowledgments
This study was supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072408 to HK), National Center for Research Resources (P20RR017659 to HK), American Heart Association (10GRNT3020018 to HK and 10GRNT 4540000 to AZ), and National Institute of Child Health and Human Development [Tulane Building Interdisciplinary Research in Women’s Health (BIRCWH), K12HD043451 to AZ]. The authors acknowledge scientific discussion and/or excellent technical assistances from L. Gabriel Navar, Ph.D.; Maki Urushihara, M.D., Ph.D.; Andrei V. Derbenev, Ph.D.; Hong Gao, Ph.D., Salem I. Elkhayat, M.S.; and Ayumi Kitano, M.S. (Tulane University).
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Eknoyan G, Hostetter T, Bakris GL, Hebert L, Levey AS, Parving HH, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK) Am J Kidney Dis. 2003;42:617–622. doi: 10.1016/s0272-6386(03)00826-6. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 6.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 7.Taguma Y, Kitamoto Y, Futaki G, Ueda H, Monma H, Ishizaki M, et al. Effect of captopril on heavy proteinuria in azotemic diabetics. N Engl J Med. 1985;313:1617–1620. doi: 10.1056/NEJM198512263132601. [DOI] [PubMed] [Google Scholar]
- 8.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The collaborative study group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 9.Nelson RG, Tuttle KR. Prevention of diabetic kidney disease: negative clinical trials with renin-angiotensin system inhibitors. Am J Kidney Dis. 2010;55:426–430. doi: 10.1053/j.ajkd.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 11.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–1961. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 12.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 13.Haller H, Ito S, Izzo JL, Jr, Januszewicz A, Katayama S, Menne J, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 14.Ingelfinger JR. Preemptive olmesartan for the delay or prevention of microalbuminuria in diabetes. N Engl J Med. 2011;364:970–971. doi: 10.1056/NEJMe1014147. [DOI] [PubMed] [Google Scholar]
- 15.Miller JA, Curtis JR, Sochett EB. Relationship between diurnal blood pressure, renal hemodynamic function, and the renin-angiotensin system in type 1 diabetes. Diabetes. 2003;52:1806–1811. doi: 10.2337/diabetes.52.7.1806. [DOI] [PubMed] [Google Scholar]
- 16.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, et al. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzaki Y, Ozawa Y, Kobori H. Intrarenal oxidative stress and augmented angiotensinogen are precedent to renal injury in Zucker diabetic fatty rats. Int J Biol Sci. 2007;3:40–46. doi: 10.7150/ijbs.3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2008;35:922–927. doi: 10.1111/j.1440-1681.2008.04938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ihara G, Kiyomoto H, Kobori H, Nagai Y, Ohashi N, Hitomi H, et al. Regression of superficial glomerular podocyte injury in type 2 diabetic rats with overt albuminuria: effect of angiotensin II blockade. J Hypertens. 2010;28:2289–2298. doi: 10.1097/HJH.0b013e32833dfcda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo TH, Li JJ, Kim JJ, Jung DS, Kwak SJ, Ryu DR, et al. Activation of the renin-angiotensin system within podocytes in diabetes. Kidney Int. 2007;71:1019–1027. doi: 10.1038/sj.ki.5002195. [DOI] [PubMed] [Google Scholar]
- 21.Kobori H, Katsurada A, Miyata K, Ohashi N, Satou R, Saito T, et al. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol. 2008;294:F1257–F1263. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S, Bivona BJ, Kobori H, Seth DM, Chappell MC, Lazartigues E, et al. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am J Physiol Renal Physiol. 2010;298:F37–F48. doi: 10.1152/ajprenal.00519.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castrop H, Hocherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev. 2010;90:607–673. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 25.Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res. 1971;5:86–89. doi: 10.1093/cvr/5.1.86. [DOI] [PubMed] [Google Scholar]
- 26.Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27:465–475. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa S, Kobori H, Ohashi N, Urushihara M, Nishiyama A, Mori T, et al. Angiotensin II type 1 receptor blockers reduce urinary angiotensinogen excretion and the levels of urinary markers of oxidative stress and inflammation in patients with type 2 diabetic nephropathy. Biomark Insights. 2009;4:97–102. doi: 10.4137/bmi.s2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito T, Urushihara M, Kotani Y, Kagami S, Kobori H. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci. 2009;338:478–480. doi: 10.1097/MAJ.0b013e3181b90c25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugimoto H, Grahovac G, Zeisberg M, Kalluri R. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes. 2007;56:1825–1833. doi: 10.2337/db06-1226. [DOI] [PubMed] [Google Scholar]
- 30.Gabra BH, Sirois P. Pathways for the bradykinin B1 receptor-mediated diabetic hyperalgesia in mice. Inflamm Res. 2004;53:653–657. doi: 10.1007/s00011-004-1310-0. [DOI] [PubMed] [Google Scholar]
- 31.Hamada Y, Kitazawa S, Kitazawa R, Fujii H, Kasuga M, Fukagawa M. Histomorphometric analysis of diabetic osteopenia in streptozotocin-induced diabetic mice: a possible role of oxidative stress. Bone. 2007;40:1408–1414. doi: 10.1016/j.bone.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Brezniceanu ML, Wei CC, Chenier I, Sachetelli S, Zhang SL, et al. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol. 2008;19:269–280. doi: 10.1681/ASN.2007010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munshi R, Johnson A, Siew ED, Ikizler TA, Ware LB, Wurfel MM, et al. MCP-1 gene activation marks acute kidney injury. J Am Soc Nephrol. 2011;22:165–175. doi: 10.1681/ASN.2010060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Li YX, Stahl GL, Thurman JM, He Y, Tong HH. Essential role of factor B of the alternative complement pathway in complement activation and opsonophagocytosis during acute pneumococcal otitis media in mice. Infect Immun. 2011;79:2578–2585. doi: 10.1128/IAI.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leehey DJ, Singh AK, Bast JP, Sethupathi P, Singh R. Glomerular renin angiotensin system in streptozotocin diabetic and Zucker diabetic fatty rats. Transl Res. 2008;151:208–216. doi: 10.1016/j.trsl.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, et al. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, et al. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun. 2007;358:156–163. doi: 10.1016/j.bbrc.2007.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292:F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozawa Y, Kobori H. Crucial role of Rho-nuclear factor-kappaB axis in angiotensin II-induced renal injury. Am J Physiol Renal Physiol. 2007;293:F100–F109. doi: 10.1152/ajprenal.00520.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75:285–294. doi: 10.1038/ki.2008.499. [DOI] [PubMed] [Google Scholar]
- 42.He Y, Martinez-Fleites C, Bubb A, Gloster TM, Davies GJ. Structural insight into the mechanism of streptozotocin inhibition of O-GlcNAcase. Carbohydr Res. 2009;344:627–631. doi: 10.1016/j.carres.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Brondum E, Nilsson H, Aalkjaer C. Functional abnormalities in isolated arteries from Goto-Kakizaki and streptozotocin-treated diabetic rat models. Horm Metab Res. 2005;37(Suppl 1):56–60. doi: 10.1055/s-2005-861370. [DOI] [PubMed] [Google Scholar]
- 44.Tzovaras V, Tsimihodimos V, Kostara C, Mitrogianni Z, Elisaf M. Aminoglycoside-induced nephrotoxicity studied by proton magnetic resonance spectroscopy of urine. Nephrol Dial Transplant. 2011;26:3219–3224. doi: 10.1093/ndt/gfr074. [DOI] [PubMed] [Google Scholar]
- 45.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito M, Kondo Y, Nakatani A, Hayashi K, Naruse A. Characterization of low dose streptozotocin-induced progressive diabetes in mice. Environ Toxicol Pharmacol. 2001;9:71–78. doi: 10.1016/s1382-6689(00)00064-8. [DOI] [PubMed] [Google Scholar]
- 47.Moriwaki Y, Inokuchi T, Yamamoto A, Ka T, Tsutsumi Z, Takahashi S, et al. Effect of TNF-alpha inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol. 2007;44:215–218. doi: 10.1007/s00592-007-0007-6. [DOI] [PubMed] [Google Scholar]
- 48.Kobori H, Ozawa Y, Suzaki Y, Prieto-Carrasquero MC, Nishiyama A, Shoji T, et al. Young Scholars Award Lecture: Intratubular angiotensinogen in hypertension and kidney diseases. Am J Hypertens. 2006;19:541–550. doi: 10.1016/j.amjhyper.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43:1251–1259. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 51.Yanagawa N, Capparelli AW, Jo OD, Friedal A, Barrett JD, Eggena P. Production of angiotensinogen and renin-like activity by rabbit proximal tubular cells in culture. Kidney Int. 1991;39:938–941. doi: 10.1038/ki.1991.117. [DOI] [PubMed] [Google Scholar]
- 52.Darby IA, Congiu M, Fernley RT, Sernia C, Coghlan JP. Cellular and ultrastructural location of angiotensinogen in rat and sheep kidney. Kidney Int. 1994;46:1557–1560. doi: 10.1038/ki.1994.445. [DOI] [PubMed] [Google Scholar]
- 53.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 54.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, et al. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem. 2010;285:41935–41946. doi: 10.1074/jbc.M110.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamiyama M, Garner MK, Farragut KM, Kobori H. The establishment of a primary culture system of proximal tubule segments using specific markers from normal mouse kidneys. Int J Mol Sci. 2012;13:5098–5111. doi: 10.3390/ijms13045098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev. 2004;25:971–1010. doi: 10.1210/er.2003-0018. [DOI] [PubMed] [Google Scholar]
- 57.Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14:274–281. doi: 10.1016/s1043-2760(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 58.Nishiyama A, Nakagawa T, Kobori H, Nagai Y, Okada N, Konishi Y, et al. Strict angiotensin blockade prevents the augmentation of intrarenal angiotensin II and podocyte abnormalities in type 2 diabetic rats with microalbuminuria. J Hypertens. 2008;26:1849–1859. doi: 10.1097/HJH.0b013e3283060efa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishiyama A, Kobori H, Konishi Y, Morikawa T, Maeda I, Okumura M, et al. Mineralocorticoid receptor blockade enhances the antiproteinuric effect of an angiotensin II blocker through inhibiting podocyte injury in type 2 diabetic rats. J Pharmacol Exp Ther. 2010;332:1072–1080. doi: 10.1124/jpet.109.158113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 61.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, et al. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:170–177. doi: 10.1093/ndt/gfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Granier C, Makni K, Molina L, Jardin-Watelet B, Ayadi H, Jarraya F. Gene and protein markers of diabetic nephropathy. Nephrol Dial Transplant. 2008;23:792–799. doi: 10.1093/ndt/gfm834. [DOI] [PubMed] [Google Scholar]
- 64.Ben Ameur R, Molina L, Bolvin C, Kifagi C, Jarraya F, Ayadi H, et al. Proteomic approaches for discovering biomarkers of diabetic nephropathy. Nephrol Dial Transplant. 2010;25:2866–2875. doi: 10.1093/ndt/gfq258. [DOI] [PubMed] [Google Scholar]
- 65.Matheson A, Willcox MD, Flanagan J, Walsh BJ. Urinary biomarkers involved in type 2 diabetes: a review. Diabetes Metab Res Rev. 2010;26:150–171. doi: 10.1002/dmrr.1068. [DOI] [PubMed] [Google Scholar]
- 66.Zhang SL, Filep JG, Hohman TC, Tang SS, Ingelfinger JR, Chan JS. Molecular mechanisms of glucose action on angiotensinogen gene expression in rat proximal tubular cells. Kidney Int. 1999;55:454–464. doi: 10.1046/j.1523-1755.1999.00271.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhang SL, Tang SS, Chen X, Filep JG, Ingelfinger JR, Chan JS. High levels of glucose stimulate angiotensinogen gene expression via the P38 mitogen-activated protein kinase pathway in rat kidney proximal tubular cells. Endocrinology. 2000;141:4637–4646. doi: 10.1210/endo.141.12.7844. [DOI] [PubMed] [Google Scholar]
- 68.Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology. 2002;143:2975–2985. doi: 10.1210/endo.143.8.8931. [DOI] [PubMed] [Google Scholar]
- 69.Zhang SL, To C, Chen X, Filep JG, Tang SS, Ingelfinger JR, et al. Essential role(s) of the intrarenal renin-angiotensin system in transforming growth factor-beta1 gene expression and induction of hypertrophy of rat kidney proximal tubular cells in high glucose. J Am Soc Nephrol. 2002;13:302–312. doi: 10.1681/ASN.V132302. [DOI] [PubMed] [Google Scholar]
- 70.Zhang SL, Chen X, Hsieh TJ, Leclerc M, Henley N, Allidina A, et al. Hyperglycemia induces insulin resistance on angiotensinogen gene expression in diabetic rat kidney proximal tubular cells. J Endocrinol. 2002;172:333–344. doi: 10.1677/joe.0.1720333. [DOI] [PubMed] [Google Scholar]
- 71.Zhang SL, Chen X, Wei CC, Filep JG, Tang SS, Ingelfinger JR, et al. Insulin inhibits dexamethasone effect on angiotensinogen gene expression and induction of hypertrophy in rat kidney proximal tubular cells in high glucose. Endocrinology. 2002;143:4627–4635. doi: 10.1210/en.2002-220408. [DOI] [PubMed] [Google Scholar]
- 72.Hsieh TJ, Fustier P, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, et al. High glucose stimulates angiotensinogen gene expression and cell hypertrophy via activation of the hexosamine biosynthesis pathway in rat kidney proximal tubular cells. Endocrinology. 2003;144:4338–4349. doi: 10.1210/en.2003-0220. [DOI] [PubMed] [Google Scholar]