Abstract
Background
Environmental factors such as inhaled pollutants like cigarette smoke may play a significant role in diseases of the upper airway including chronic rhinosinusitis (CRS). The objectives of this review are to summarize prior studies that describe the correlation between active smoking and second-hand smoke (SHS) on CRS. We also review the pathophysiologic effects of cigarette smoke on sinonasal mucosa and discuss its impact on surgical outcomes of endoscopic sinus surgery (ESS).
Methods
A literature search was conducted with the PUBMED database using the terms “sinusitis” or “rhinosinusitis” and “smoking”. Additional search terms of “nasal epithelial” and “smoke” were used to find articles which discussed pathophysiologic effects of tobacco smoke while “second-hand smoke” was added to identify articles analyzing the correlation of SHS and CRS. Finally “endoscopic sinus surgery” and “outcomes” were linked to “smoking” to find articles that analyzed the impact of smoking on surgical results.
Results
204 articles were identified in the initial search. An additional 72 articles were reviewed for their relevance to the pathophysiologic effects of tobacco smoke while 31 papers were analyzed to determine the correlation of SHS and CRS. Twenty-nine papers were reviewed to analyze the impact of smoking on surgical results.
Conclusion
There is clear evidence in the literature that cigarette smoke, either through active smoking or passive exposure to SHS, contributes to CRS. Recent prospective studies suggest that active smoking is not a contraindication to ESS while the impact of smoking volume and long-term smoking after ESS has not been sufficiently evaluated.
Keywords: sinusitis, etiology, tobacco, smoking, tobacco smoke pollution, endoscopic surgical procedure, outcome assessment, sinonasal, epithelial cells
Introduction
Chronic rhinosinusitis (CRS) is a prevalent condition that has a significant health and economic impact. There are numerous proposed etiologies of CRS which include alterations in innate immunity, inflammatory dysregulation, biofilms, and super-antigen effects. Sinonasal epithelial cells serve as a mechanical and immunologic barrier and act as a frontline defense against inhaled pollutants, irritants and toxins. Environmental factors such as allergens and inhaled pollutants such as cigarette smoke may play a significant role in diseases of the upper airway including asthma, otitis media, and rhinosinusitis. There have been several studies that have investigated the effects of active tobacco smoking as well as exposure to second-hand smoke (SHS) on the upper airway and their association with upper airway diseases. The objective of this review is to outline the existing data on the correlation between active smoking and SHS on the etiology of CRS. We will review the specific impact of direct and indirect tobacco smoke on the immunologic and mechanical function of sinonasal epithelial cells to better describe their pathophysiologic effects on the upper airway. Additionally we will discuss and attempt to provide some clarity on the conflicting literature regarding the impact of smoking on outcomes after ESS.
The Correlation of Tobacco Smoke Exposure and Rhinosinusitis
The upper airway functions as the first line of defense against inhaled toxins and pathogens including environmental pollutants such as tobacco smoke as well as microbes that can cause potential harm to the host. The adverse effects of tobacco smoke on the upper airway are well described in the literature. Much of the previous reports have focused on the impact of SHS on adults and children. Various health organizations have found an association with SHS and an increased incidence of upper airway disease in children.1 Prenatal maternal smoking has been shown to be associated with early childhood wheezing and asthma.2–4 Additionally, exposure to SHS in the home has also been shown to increase the risk and severity of childhood asthma.4–5 Gergen et al. analyzed the information from the third National Health and Nutrition Examination Survey (NHANES III) to look at the impact of environmental tobacco smoke on children between 2 months and 5 years old.6 The authors found that the prevalence of wheezing and asthma was increased in children exposed to SHS with an adjusted odds ratio of 2.1 attributable to an additional 133,800 to 161,600 cases. In addition to its impact on the upper airway, SHS has also been shown to increase the risk of otitis media and middle ear disease by approximately 50%.7
A PUBMED search using the terms “sinusitis” or “rhinosinusitis” and “smoking” yields 238 articles. Limiting these to English language articles eliminates 34 articles. The remaining 204 articles were reviewed for relevance, specifically articles that analyzed the correlation of active tobacco smoking with the prevalence of adult sinusitis. For each search conducted in this study, abstracts were evaluated, and full-length articles were reviewed if the abstract was deemed relevant to the topic. References were hand-searched for additional relevant articles. In this search, three population based studies were found. Lieu et al. analyzed the NHANES III study to correlate both active and passive tobacco smoke exposure with the prevalence of sinusitis.8 The authors quantified degree of tobacco exposure by assessing reported number of cigarettes smoked, past cigarette smoking, as well as number of household smokers and number of cigarettes they smoked in the home. Tobacco exposure dose was also quantified in the 60% of included respondents for whom serum cotinine were available. The authors found that the prevalence of sinusitis increased in a dose dependant manner (when including serum cotinine levels) with participants who smoked more than 40 cigarettes having an adjusted risk ratio of 1.20. Current smokers reported an increased prevalence of both acute sinusitis (RR, 1.18) and recurrent or chronic sinusitis (RR, 1.22). A major limitation of this paper was that the presence of sinusitis was self-reported in the NHANES III respondents. Certainly rhinitis, headaches or migraines and anatomic nasal septal abnormalities can masquerade as sinusitis and creates the potential for significant error in determining the true prevalence of sinusitis in the study population.
Other studies looking at national population survey statistics have yielded similar findings. Chen et al. used data from the National Population Health Survey, a Canadian health survey distributed to 95,466 households in 10 provinces, to study the prevalence and risk factors for CRS.9 Survey respondents older than 12 years of age who answered affirmatively to the question “do you have sinusitis diagnosed by a health professional?” were included in the study. Smoking was positively associated with CRS in women with and without a history of allergy. However, in male subjects smoking only correlated with a higher prevalence of CRS in subject without a history of allergy. Women in this study had a higher prevalence of CRS than men (5.7% vs. 3.4%). The Global Allergy and Asthma European Network (GA2LEN) survey also looked at patients with CRS.10 CRS was defined as a reported history of greater than 12 weeks of two or more symptoms including either nasal blockage/obstruction/congestion or nasal drainage. There was a small but significant association of CRS with being an ex-smoker (1.28, 95% CI 1.18 – 1.38) and a strong association of CRS with active smoking (1.91, 95% CI 1.77 – 2.05). Similar to the NANES III study, both the GA2LEN and Canadian studies are limited by the fact that the determination of CRS was self reported and therefore may overestimate the true prevalence of the condition. Certainly smoking by itself may generate similar sinonasal symptoms that could mimic CRS. Houser and Keen performed a retrospective analysis of CRS patients who underwent ESS and found a significant association between direct tobacco use and nasal polyps.11 Interestingly, they also found that higher levels of smoking in terms of packs per day were actually protective against polyps. This study is limited in that it is a retrospective analysis of a small population of patients with CRS undergoing sinus surgery and relies on a comparison of smoking prevalence in the general population for its analysis.
Pathophysiologic Effects of Tobacco Smoke on Sinonasal Mucosa
Many studies have examined the pathophysiologic effects of tobacco smoke on sinonasal mucosa in an effort to explain the biologic rationale for the clinical association of smoke and CRS. In addition to the aforementioned search which yielded 204 articles, an additional search was conducted using the terms “nasal epithelial” and “smoke” yielded 72 articles. Limiting these articles for English language resulted in 70 articles which were reviewed for relevance. Some of these studies have documented the impact of smoke on nasal physiology and function. Willes et al. analyzed nasal symptoms and airflow response of 18 individuals with a reported history of tobacco smoke sensitivity by exposing them to sidestream smoke, the major component of second-hand smoke (SHS).12 Although the study lacked an appropriate control group, eye irritation, nasal irritation, nasal congestion, and rhinorrhea were the most frequent symptoms after smoke exposure. Increased nasal airway resistance and reduced maximum inspiratory flow were also observed after exposure to sidestream tobacco smoke.
Sinonasal epithelial cells utilize mucociliary clearance (MCC) which relies on an airway surface liquid (ASL) barrier and proper ciliary beating to effectively transport mucus and clear potentially toxic irritants. Prior experiments have shown that tobacco smoke can adversely affect MCC. Zhou et al. analyzed the ciliary beat frequency (CBF) in biopsy samples of 61 patients; 21 non-smokers, 27 smokers, and 13 non-smokers who were regularly exposed to SHS. They found that CBF was increased in smokers and non-smokers exposed to high levels of SHS as compared to non-smokers.13 The authors also analyzed CBF in air-liquid interface cell cultures from 5 non-smokers and found that when exposed to high concentrations of a cigarette smoke condensate (CSC) (100μg/ml) there was a corresponding increase in CBF. Another study analyzed changes in mucociliary clearance after exposure to sidestream smoke in 6 individuals with and 6 individuals without a history of SHS sensitivity.14 While most individuals showed increased mucociliary clearance after exposure to sidestream smoke compared to clean air exposure, 3 individuals with a history of SHS sensitivity experienced reduced mucociliary clearance.
Cohen et al. analyzed both murine nasal septal epithelial and human sinonasal epithelial cultures to determine the effects of CSC (200μg/ml) on transepithelial Cl− secretion and CBF.15 The ASL is maintained by vectorial transport of various ions including Cl−. In the disease cystic fibrosis in which the Cl− is disrupted, the ASL function becomes impaired causing chronic stasis of inspissated mucus leading to bacterial infections and CRS. The authors found that CSC inhibits both Cl− transport as well as decreases stimulated CBF in both murine and human sinonasal epithelial cell cultures. Tamashiro et al. also found that CSC impaired ciliogenesis in a dose dependent manner in murine sinonasal epithelial cells cultures.16
At first glance, these studies provide some conflicting information. However Zhou et al. 13 used lower concentrations of CSC than those used by Cohen et al. 15 It is possible, as Zhou suggests in his paper, that while lower concentrations of tobacco smoke acts as a compensatory stimulant of MCC, higher exposure causes pathologic changes to sinonasal epithelial cells that serves to adversely reduce MCC function. In a study of 23 individuals grouped by their prior SHS sensitivity, both SHS sensitive and non-sensitive individuals had increased symptoms of rhinorrhea, nasal congestion, and headache following sidestream smoke exposure.17 Individuals with known tobacco smoke sensitivity had more severe symptoms than those without previous history of SHS related rhinitis. This study suggests that certain people may be predisposed to the effects of tobacco smoke in terms of increased sinonasal physiological and symptom response. Patients with sensitivity to tobacco smoke may be at higher risk for developing chronic upper respiratory inflammation and disease, such as CRS, in response to SHS.
The innate immune function of sinonasal epithelium has become a significant area of research as a potential cause of CRS. These studies have shown alterations in locally expressed pattern receptors such as toll-like receptors (TLRs) and innate immune effector proteins such as β-defensins and complement components in CRS sinonasal epithelium.18–19 Prior studies have shown that tobacco smoke has immunosuppressive effects by suppressing monocyte-derived macrophage function as well as by inhibiting inflammatory cytokines by suppressing TLR mediated pathways in human bronchial epithelial cells.20 Lee et al. found that acrolein, a volatile component of tobacco smoke, inhibits the cytokine IL-8 and human β-defensin (HBD-2) in sinonasal epithelial cell cultures derived from patients with CRS.20 IL-8 is a cytokine involved in neutrophil recruitment while HBD-2 is an effector molecule that is effective in killing gram negative bacteria and has a bacteriostatic effect on gram positive bacteria such as Staphylococcus and Streptococcus. These findings suggest that cigarette smoke may have a suppressive function on sinonasal innate immunity. Yamin et al. found that cigarette smoke extract (CSE) combined with dsRNA, a viral pathogen, selectively induces exaggerated RANTES and HBD-2 expression in CRS epithelium in vitro as compared to normal control epithelium.21 The role of RANTES in epithelial innate immunity is uncertain, although increased expression of this cytokine has been demonstrated in asthma22 and nasal polyps.23
Yee et al. compared biopsies of olfactory respiratory epithelium from both smoking and non-smoking CRS patients without nasal polyps.24 They found that the relative presence of normal pseudostratified epithelium and goblet cell hyperplasia tended to decrease with increased patient exposure to tobacco smoke while squamous metaplasia increased. Along with the increased degree of squamous metaplasia, the olefactory sensory neuron morphology became increasingly abnormal with increased exposure to tobacco smoke. In a multi-institutional study of the olfactory function of 369 CRS patients, Litvack et al. found that the presence of nasal polyps and smoking correlated with anosmia.25 While this study did not quantify the number or frequency of cigarettes, the data in this and other studies26 supports that tobacco smoke may adversely impact olfaction.
The Relationship of Second-hand Smoke and Rhinosinusitis
Until recently the epidemiologic association between SHS and CRS has not been well characterized. A PUBMED search was conducted with the search terms “second-hand smoke” and “sinusitis” which yielded 33 papers, 31 of which were written in the English language. These papers were selected for those which specifically analyzed the relationship between SHS and sinusitis. In the US, the National Health and Nutrition Examination Survey (NHANES) found that household SHS exposure was also associated with sinusitis, although the association was not statistically significant (adjusted odds ratio 1.15; 95% CI 0.98, 1.32).8 NHANES was not specifically designed to evaluate the association of SHS with CRS. Recently, two case-control studies, conducted in Washington County, Maryland27 and Detroit, Michigan28 were performed to specifically evaluate the relationship of SHS exposure with CRS (Table 1)..
Table 1.
Characteristics of case control studies on secondhand smoke (SHS) exposure and chronic rhinosinusitis (CRS)
| 1st author, year | Location | Population | % Men | Age, mean (SD) | CRS assessment | Cases / Controls | SHS assessment | OR (95% CI) | Matched for | Adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Tammemagi, 2010 | Detroit, Michigan | Never and former smokers in Henry Ford Health System for ≥5 y | 44.1 | 53.8 (14.5) | ICD-9 codes 473 and 471 + CT or nasal endoscopy confirmation | 306 / 306 | Exposure in past 5 years: | Age, sex and race/ethnicity | Education, other SHS sources and self- reported exposure to air pollution, chemicals and irritants | |
| Home (any) | 1.85 (0.98, 3.51) | |||||||||
| Work (any) | 2.57 (1.26, 5.27) | |||||||||
| Public (>10 times/mo.) | 1.27 (0.76, 2.13) | |||||||||
| Private (any) | 2.49 (1.65, 3.76) | |||||||||
| Any of above | 2.20 (1.51, 3.20) | |||||||||
|
| ||||||||||
| Reh, 2009 | Washington County, Maryland | Never and >2y former smokers from population- based cohort followed yearly since 1996 (n=7,386) | 31.5 | 59.9 (10.9) | Sinonasal symptom(s) for > 3 months + CT or nasal endoscopy confirmation* | 100 / 100 | Current (living with smoker or ≥1 h/wk in any private or public place) | 2.31 (0.66, 8.07) | Age, sex and never/former smoking | Education |
| Childhood (living with a smoker) | 2.28 (0.96, 6.34) | |||||||||
| Current or childhood | 2.33 (1.02, 5.34) | |||||||||
Reproduced from: Reh DD, Navas-Acien A. The relationship between secondhand tobacco smoke exposure and chronic rinosinusitis: Evidence of causality. Expert Rev Respir Med. 2010; 4(4):445–9. CI, confidence interval; CT, computed tomography; ICD-9, International classification of diseases 9th revision; mo: month; OR, odds ratio; SD, standard deviation; wk, week; y, year.
Physician confirmation available for 78% of the cases (for 22% only self-reported physician confirmation available)
Reh et al., conducted a study within a population-based prospective cohort study that has followed more than 7000 individuals on a yearly basis in Washington County, MD. This study matched for never/former smoking status to control for potential confounding induced by past active smoking. To remove any contribution from recent active smoking, former smokers had to have refrained from smoking for at least 2 years. This study defined CRS using the Rhinosinustis Task Force definition29 which included the presence of sinonasal symptoms for more than 12 weeks and as well as sinonasal inflammation confirmed by computer tomography, rhinoscopy or nasal endoscopy. Physician diagnosis was confirmed in 78% of the cases. Current SHS exposure was based on a questionnaire validated with hair nicotine concentrations.30
The study by Tammemagi et al. was an age, sex and race/ethnicity matched case-control study nested within a well defined population: a health care system in Detroit, Michigan which is similar to the general population of Detroit (Table 1).28 Enrolled patients participated in the health care system for more than 5 years (without a CRS diagnosis for cases) and were never or former smokers (quit length not specified). Cases were selected if they had a CRS related diagnosis code (International Classification of Diseases, Ninth Revision9 code 473 for chronic sinusitis and 471 for nasal polyps) between January 1, 2000 and May 1, 2004 confirmed by computer tomography or nasal endoscopy. While the authors indicated that, when possible, the case definition followed the diagnostic criteria recommended by the Rhinosinusitis Task Force, information on duration of sinonasal symptoms for at least 12 weeks was not obtained. Controls were required to be free of CRS for at least 5 years prior to study enrollment.
Both studies had similar findings, supporting the role of SHS exposure in the development of CRS. In Tammemagi et al., exposure to SHS at home, work, public places (at least 10 times/month) or private places during the past 5 years was associated with an increased risk of CRS (odds ratio 2.20, 95% confidence interval 1.51, 3.20).28 The associations remained similar before and after adjustment for other factors (education, air pollution and chemical exposures). In Reh et al., current or childhood exposure to SHS was associated with an increased risk of CRS (odds ratio 2.33, 95% confidence interval 1.02, 5.34).27 Current and childhood exposure to SHS were also independently associated with CRS, although not statistically significant.
This suggestive causal relationship between SHS and CRS has important practical implications. Physicians who manage the treatment and care of patients with diseases of the upper airway, including CRS, need to request information on SHS exposure in the home and other private/public environments. This information can be used to council patients with CRS and their families to avoid and eliminate SHS exposure as part of their treatment. Additionally, public health strategies to reduce or eliminate SHS exposure in public and private places may have a major impact in the prevention and control of CRS. While the impact of reducing public SHS on the prevalence of CRS has not been formally evaluated, between 1997 and 2007, parallel to a marked decrease in SHS exposure31–32, the prevalence of self-reported physician diagnosis of sinusitis in US adults decreased from 16% to 11% (Figure 1).33 It is important to note that the National Health Interview Survey from which these numbers are derived does not differentiate between acute sinusitis and CRS.
Figure 1.
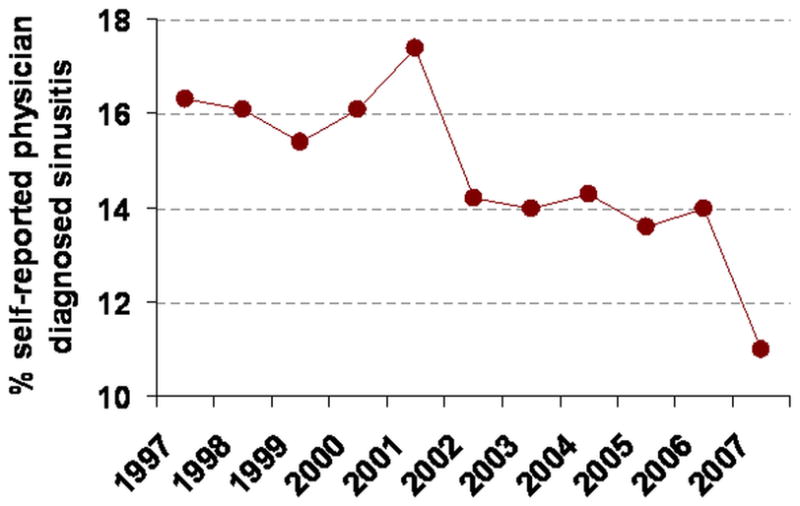
Prevalence of self-reported physician diagnosis of sinusitis in U.S. adults based on the National Health Interview Survey (Data derived from http://www.cdc.gov/nchs/products/series/series10.htm). Reproduced from: Reh DD, Lin SY, ClippS, IraniL, AlbergA, Navas-Acien A. Secondhand Tobacco Smoke Exposure and Chronic Rhinosinusitis: A Case-Control Study. Am J Rhinol Allergy. 2009; 23(6): 562–567.
The Impact of Tobacco Smoke on Endoscopic Sinus Surgery Outcomes
A PUBMED search was conducted with the search terms “smoking” and “sinus surgery” which yielded 29 papers, all of which were written in the English language. Surgical outcomes studies evaluating FESS for CRS in active smokers have yielded opposing results. Table 2 lists the different findings of multiple prospective and retrospective studies that have reviewed the impact of tobacco smoke exposure on ESS clinical outcomes. Retrospective studies in the 1990s found deleterious effects of active smoking on surgical outcomes, including poorer symptom scores34, worse patient-reported outcomes35, and an observation that revision sinus surgery cases had a higher proportion of smokers than primary sinus surgery cases (27% versus 10%)36. Later retrospective studies reported less olfactory37 and poorer health related quality of life (HRQOL)38 improvement in smokers compared to nonsmokers. A subsequent series of prospective cohort studies, however, has challenged these findings. A small prospective study that found no difference in postoperative endoscopy scores was followed by larger prospective cohort studies that found similar outcomes between smokers and nonsmokers in not only endoscopy scores39–43, but also HRQOL40, 42–44 and olfactory improvement. One study of 274 patients did find a statistically significant higher rate of revision ESS in smokers versus nonsmokers.44
Table 2.
Literature summary of studies evaluating the effect of smoking on ESS outcomes
| Author | Year | Study type | Total No. CRS pts. | Total no. of Smoking pts. | Report Smoking Volume | Mean F/U (years) | Clinical outcome | Results |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Studies demonstrating worse ESS outcomes in smokers with CRS (n=5)
| ||||||||
| Danielsen et al.26 | 1996 | Retrospective | 230 | 74 | No | 3.5 | Symptoms score rating 1 to 5 | Smoking reduced post-op ESS symptom scores |
|
| ||||||||
| Senior et al.11 | 1998 | Retrospective | 72 | 14 | No | 7.8 | Revision ESS | Higher number of smokers in revision ESS group (27% vs. 10%) |
|
| ||||||||
| Sobol et al.27 | 1998 | Retrospective | 274 | 43 | No | 1 | Pt. reported outcome | Smokers reported poorer outcome at 12 months (57.2% vs. 27.6%) |
|
| ||||||||
| Sugiyama et al.28 | 2002 | Retrospective | 37 | 13 | No | Not stated | Olfactory function | Less olfactory improvement after ESS in smokers age > 40 |
|
| ||||||||
| Briggs et al.12 | 2004 | Retrospective | 82 | 26 | Yes | 4.3 | HRQoL | Smoking is associated with reduced HRQoL improvement |
|
| ||||||||
| Studies demonstrating no difference in ESS outcomes in smokers with CRS (n=6)
| ||||||||
| Watelet et al.29 | 2004 | Prospective | 36 | 7 | No | 0.5 | Postop endoscopy | No difference in Post-op endoscopy |
|
| ||||||||
| Smith et al.13 | 2005 | Prospective | 119 | 11 | No | 1.4 | Postop endoscopy | No difference in Post-op endoscopy |
| HRQoL | Smokers had the same HRQoL benefit as non- smokers | |||||||
|
| ||||||||
| Das et al.25 | 2007 | Prospective | 221 | 50 | No | 0.25 | Postop endoscopy | No difference in Post-op endoscopy |
| HRQoL | Smokers experienced more HRQoL improvement at 3 months | |||||||
|
| ||||||||
| Danielides et al.30 | 2009 | Prospective | 116 | 44 | Yes | 0.5 | Olfactory function | No difference in olfactory improvement rates between smokers (who quit post-ESS) and non-smokers |
|
| ||||||||
| Das et al.14 | 2009 | Prospective | 116 | 26 | No | 3.6 | Postop endoscopy | Smokers had stable endoscopic and HRQoL improvement at long-term follow-up |
| HRQoL | ||||||||
|
| ||||||||
| Rudmik et al. (current study) | 2010 | Prospective | 712 | 39 | Yes | 1.4 | Postop endoscopy | No difference in HRQoL outcomes between smokers and non-smokers |
| HRQoL | Heavy smokers demonstrated worsened endoscopic appearance | |||||||
Reproduced from: Rudmik L, Mace JC, Smith TL. Smoking and endoscopic sinus surgery: does smoking volume contribute to clinical outcome? Int Forum Allergy Rhinol. 2011; 1(2):145–152. CRS, chronic rhinosinusistis; F/U, follow-up; ESS, endoscopic sinus surgery; HRQoL, health-related quality of life.
Volume of smoking seems to be an important factor in outcomes. A prospective, multi-institutional cohort study of 784 subjects managed at 3 rhinology clinics in the United States demonstrated that high volume smokers (defined as over 20 cigarettes a day) had statistically significantly worse postoperative endoscopy scores than low volume smokers (less than or equal to 20 cigarettes a day) or nonsmokers. Interestingly, high volume smoking was not associated with any difference in HRQOL.42
The reason for the disparity in results is unclear. One explanation is the difference in study design since retrospective studies can have several outcome biases, such as incomplete information, inconsistent measurements, investigator bias, and variable follow-up. Another explanation may be related to temporal differences in smoking habits. From 1998 to 2008, the Center for Disease Control (CDC) reported a decrease in the proportion of United States adults who currently smoked had decreased 3.5% (from 24.1% to 20.6%).45 The proportion of heavy smoking, defined as greater than or equal to 25 cigarettes per day, decreased from 19.1% of smokers in 1993 to 12.1% of smokers in 2004.46 Therefore, early surgical outcome studies may have seen positive results because these studies included more heavy smokers than subsequent studies.
Surgical outcomes with SHS exposure have been mostly studied in children. A cohort study of 38 children with CRS between 4 and 17 years of age were followed for ciliary regeneration after ESS. Biopsies were performed of the maxillary sinus osteum at the time of surgery and 5 to 10 months after surgery. In children with no parental-reported SHS exposure, the mean ciliary area increased from 26.6% to 35.1% of total epithelial surface (n = 14, P < 0.001); whereas, the ciliary area did not demonstrate a statistically significant improvement in children exposed to SHS (9.9% to 10.5%, n = 24, P = 0.55). The difference in change between these groups was statistically significant (P < 0.001).47 Two population-based studies found that symptom improvement after ESS was less in children with parental-reported second-hand smoke exposure.48–49
Both active cigarette smoking and SHS exposure appears to have a negative impact on endoscopic examination after sinus surgery and rate of revision surgery with volume of smoking being a significant contributing factor; however, HRQOL seems to be similar between smokers and nonsmokers.
Conclusion
In this review, we provide an overview of the literature on the association between tobacco smoke exposure and CRS. Prior population based studies from the U.S. and Europe have shown a likely correlation between active use of cigarette smoke and the development of CRS. These studies also suggest a dose dependant effect in terms of the number of cigarettes and the prevalence of CRS but are limited by the fact that the determination of CRS was based on self reporting and therefore may overestimate the effect of smoke exposure.
Multiple studies have analyzed the pathophysiologic effects of tobacco smoke on sinonasal mucosa. Cigarette smoke induces a physiologic nasal response including increased nasal airway resistance, nasal irritation, nasal congestion, and rhinorrhea. Tobacco smoke extract has been demonstrated to have adverse effects on sinonasal epithelial mucociliary clearance and innate immune function as well as olfactory mucosal metaplasia. Two recent case control studies have shown that SHS exposure has a causal relationship with CRS. Recent prospective studies suggest that active smoking may not be a contraindication to ESS while the impact of smoking volume and long-term smoking after ESS has not been sufficiently evaluated. Future prospective studies that quantify volume of daily tobacco smoke exposure are required in order to determine whether there is a dose dependant effect on ESS outcomes.
Footnotes
Conflict of Interest: None
Financial Disclosures: None for Douglas D. Reh or Thomas S. Higgins. Timothy L. Smith is a research consultant for Intersect ENT (Palo Alto, CA) and is funded by the National Institute on Deafness and Other Communication Disorders, one of the National Institutes of Health (Bethesda, MD).
References
- 1.Health effects of exposure to environmental tobacco smoke. California Environmental Protection Agency. Tob Control. 1997 Winter;6(4):346–353. doi: 10.1136/tc.6.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis S, Richards D, Bynner J, Butler N, Britton J. Prospective study of risk factors for early and persistent wheezing in childhood. Eur Respir J. 1995 Mar;8(3):349–356. doi: 10.1183/09031936.95.08030349. [DOI] [PubMed] [Google Scholar]
- 3.Tariq SM, Hakim EA, Matthews SM, Arshad SH. Influence of smoking on asthmatic symptoms and allergen sensitisation in early childhood. Postgrad Med J. 2000 Nov;76(901):694–699. doi: 10.1136/pmj.76.901.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein RT, Holberg CJ, Sherrill D, et al. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson Children’s Respiratory Study. Am J Epidemiol. 1999 Jun 1;149(11):1030–1037. doi: 10.1093/oxfordjournals.aje.a009748. [DOI] [PubMed] [Google Scholar]
- 5.Dezateux C, Stocks J, Dundas I, Fletcher ME. Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med. 1999 Feb;159(2):403–410. doi: 10.1164/ajrccm.159.2.9712029. [DOI] [PubMed] [Google Scholar]
- 6.Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics. 1998 Feb;101(2):E8. doi: 10.1542/peds.101.2.e8. [DOI] [PubMed] [Google Scholar]
- 7.Strachan DP, Cook DG. Health effects of passive smoking. 4. Parental smoking, middle ear disease and adenotonsillectomy in children. Thorax. 1998 Jan;53(1):50–56. doi: 10.1136/thx.53.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieu JE, Feinstein AR. Confirmations and surprises in the association of tobacco use with sinusitis. Arch Otolaryngol Head Neck Surg. 2000 Aug;126(8):940–946. doi: 10.1001/archotol.126.8.940. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Dales R, Lin M. The epidemiology of chronic rhinosinusitis in Canadians. Laryngoscope. 2003 Jul;113(7):1199–1205. doi: 10.1097/00005537-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe--an underestimated disease. A GA(2)LEN study. Allergy. 2011 Sep;66(9):1216–1223. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 11.Houser SM, Keen KJ. The role of allergy and smoking in chronic rhinosinusitis and polyposis. Laryngoscope. 2008 Sep;118(9):1521–1527. doi: 10.1097/MLG.0b013e31817d01b8. [DOI] [PubMed] [Google Scholar]
- 12.Willes SR, Fitzgerald TK, Bascom R. Nasal inhalation challenge studies with sidestream tobacco smoke. Arch Environ Health. 1992 May-Jun;47(3):223–230. doi: 10.1080/00039896.1992.9938353. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Wang X, Brighton L, Hazucha M, Jaspers I, Carson JL. Increased nasal epithelial ciliary beat frequency associated with lifestyle tobacco smoke exposure. Inhal Toxicol. 2009 Aug;21(10):875–881. doi: 10.1080/08958370802555898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bascom R, Kulle T, Kagey-Sobotka A, Proud D. Upper respiratory tract environmental tobacco smoke sensitivity. Am Rev Respir Dis. 1991 Jun;143(6):1304–1311. doi: 10.1164/ajrccm/143.6.1304. [DOI] [PubMed] [Google Scholar]
- 15.Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009 Nov;119(11):2269–2274. doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 16.Tamashiro E, Xiong G, Anselmo-Lima WT, Kreindler JL, Palmer JN, Cohen NA. Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am J Rhinol Allergy. 2009 Mar-Apr;23(2):117–122. doi: 10.2500/ajra.2009.23.3280. [DOI] [PubMed] [Google Scholar]
- 17.Willes SR, Fitzgerald TK, Permutt T, Proud D, Haley NJ, Bascom R. Acute respiratory response to prolonged, moderate levels of sidestream tobacco smoke. J Toxicol Environ Health A. 1998 Feb 6;53(3):193–209. doi: 10.1080/009841098159330. [DOI] [PubMed] [Google Scholar]
- 18.Lane AP, Truong-Tran QA, Myers A, Bickel C, Schleimer RP. Serum amyloid A, properdin, complement 3, and toll-like receptors are expressed locally in human sinonasal tissue. Am J Rhinol. 2006 Jan-Feb;20(1):117–123. [PMC free article] [PubMed] [Google Scholar]
- 19.Vandermeer J, Sha Q, Lane AP, Schleimer RP. Innate immunity of the sinonasal cavity: expression of messenger RNA for complement cascade components and toll-like receptors. Arch Otolaryngol Head Neck Surg. 2004 Dec;130(12):1374–1380. doi: 10.1001/archotol.130.12.1374. [DOI] [PubMed] [Google Scholar]
- 20.Lee WK, Ramanathan M, Jr, Spannhake EW, Lane AP. The cigarette smoke component acrolein inhibits expression of the innate immune components IL-8 and human beta-defensin 2 by sinonasal epithelial cells. Am J Rhinol. 2007 Nov-Dec;21(6):658–663. doi: 10.2500/ajr.2007.21.3094. [DOI] [PubMed] [Google Scholar]
- 21.Yamin M, Holbrook EH, Gray ST, et al. Cigarette smoke combined with Toll-like receptor 3 signaling triggers exaggerated epithelial regulated upon activation, normal T-cell expressed and secreted/CCL5 expression in chronic rhinosinusitis. J Allergy Clin Immunol. 2008 Dec;122(6):1145–1153. e1143. doi: 10.1016/j.jaci.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Castro M, Bloch SR, Jenkerson MV, et al. Asthma exacerbations after glucocorticoid withdrawal reflects T cell recruitment to the airway. Am J Respir Crit Care Med. 2004 Apr 1;169(7):842–849. doi: 10.1164/rccm.200208-960OC. [DOI] [PubMed] [Google Scholar]
- 23.Beck LA, Stellato C, Beall LD, et al. Detection of the chemokine RANTES and endothelial adhesion molecules in nasal polyps. J Allergy Clin Immunol. 1996 Oct;98(4):766–780. doi: 10.1016/s0091-6749(96)70126-4. [DOI] [PubMed] [Google Scholar]
- 24.Yee KK, Pribitkin EA, Cowart BJ, et al. Smoking-associated squamous metaplasia in olfactory mucosa of patients with chronic rhinosinusitis. Toxicol Pathol. 2009;37(5):594–598. doi: 10.1177/0192623309338055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy. 2009 Mar-Apr;23(2):139–144. doi: 10.2500/ajra.2009.23.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990 Mar 2;263(9):1233–1236. [PubMed] [Google Scholar]
- 27.Reh DD, Lin SY, Clipp SL, Irani L, Alberg AJ, Navas-Acien A. Secondhand tobacco smoke exposure and chronic rhinosinusitis: a population-based case-control study. Am J Rhinol Allergy. 2009 Nov-Dec;23(6):562–567. doi: 10.2500/ajra.2009.23.3377. [DOI] [PubMed] [Google Scholar]
- 28.Tammemagi CM, Davis RM, Benninger MS, Holm AL, Krajenta R. Secondhand smoke as a potential cause of chronic rhinosinusitis: a case-control study. Arch Otolaryngol Head Neck Surg. 2010 Apr;136(4):327–334. doi: 10.1001/archoto.2010.43. [DOI] [PubMed] [Google Scholar]
- 29.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003 Sep;129(3 Suppl):S1–32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 30.Irani L, Lin SY, Clipp SL, Alberg AJ, Navas-Acien A. Involving stakeholders to optimize a study protocol on secondhand tobacco smoke and chronic rhinosinusitis in adults. Am J Rhinol Allergy. 2010 Jan-Feb;24(1):39–44. doi: 10.2500/ajra.2010.24.3369. [DOI] [PubMed] [Google Scholar]
- 31.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006 Jun;114(6):853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Disparities in secondhand smoke exposure--United States, 1988–1994 and 1999–2004. MMWR Morb Mortal Wkly Rep. 2008;57:744–747. [PubMed] [Google Scholar]
- 33.National Health Interview Survey. [Accessed 6-1-201];Summary Health Statistics for the US population: National Health Interview Survey 1997–2007. 2009 http://www.cdc.gov/nchs/products/series.htm#sr10.
- 34.Danielsen A, Olofsson J. Endoscopic endonasal sinus surgery. A long-term follow-up study. Acta Otolaryngol. 1996 Jul;116(4):611–619. doi: 10.3109/00016489609137898. [DOI] [PubMed] [Google Scholar]
- 35.Sobol SE, Wright ED, Frenkiel S. One-year outcome analysis of functional endoscopic sinus surgery for chronic sinusitis. J Otolaryngol. 1998 Oct;27(5):252–257. [PubMed] [Google Scholar]
- 36.Senior BA, Kennedy DW, Tanabodee J, Kroger H, Hassab M, Lanza D. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998 Feb;108(2):151–157. doi: 10.1097/00005537-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama K, Matsuda T, Kondo H, et al. Postoperative olfaction in chronic sinusitis: smokers versus nonsmokers. Ann Otol Rhinol Laryngol. 2002 Nov;111(11):1054–1058. doi: 10.1177/000348940211101119. [DOI] [PubMed] [Google Scholar]
- 38.Briggs RD, Wright ST, Cordes S, Calhoun KH. Smoking in chronic rhinosinusitis: a predictor of poor long-term outcome after endoscopic sinus surgery. Laryngoscope. 2004 Jan;114(1):126–128. doi: 10.1097/00005537-200401000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Das S, Becker AM, Perakis H, Prosser JD, Kountakis SE. The effects of smoking on short-term quality of life outcomes in sinus surgery. Laryngoscope. 2007 Dec;117(12):2229–2232. doi: 10.1097/MLG.0b013e318145388f. [DOI] [PubMed] [Google Scholar]
- 40.Das S, Khichi SS, Perakis H, Woodard T, Kountakis SE. Effects of smoking on quality of life following sinus surgery: 4-year follow-up. Laryngoscope. 2009 Nov;119(11):2284–2287. doi: 10.1002/lary.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naidoo Y, Wen D, Bassiouni A, Keen M, Wormald PJ. Long-term results after primary frontal sinus surgery. Int Forum Allergy Rhinol. 2012 Jan 17; doi: 10.1002/alr.21015. [DOI] [PubMed] [Google Scholar]
- 42.Rudmik L, Mace JC, Smith TL. Smoking and Endoscopic Sinus Surgery: Does smoking volume contribute to clinical outcome? Int Forum Allergy Rhinol. 2011;1(2):145–152. doi: 10.1002/alr.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith TL, Mendolia-Loffredo S, Loehrl TA, Sparapani R, Laud PW, Nattinger AB. Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2005 Dec;115(12):2199–2205. doi: 10.1097/01.mlg.0000182825.82910.80. [DOI] [PubMed] [Google Scholar]
- 44.Krzeski A, Galewicz A, Chmielewski R, Kisiel M. Influence of cigarette smoking on endoscopic sinus surgery long-term outcomes. Rhinology. 2011 Dec;49(5):577–582. doi: 10.4193/Rhino.10.038. [DOI] [PubMed] [Google Scholar]
- 45.Center for Disease Control (CDC) Cigarette smoking among adults and trends in smoking cessation-United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(44):1227–32. [PubMed] [Google Scholar]
- 46.CDC. Cigarette smoking among adults -United States, 2004. MMWR. 2005;54(44):1221–24. [PubMed] [Google Scholar]
- 47.Atef A, Zeid IA, Qotb M, El Rab EG. Effect of passive smoking on ciliary regeneration of nasal mucosa after functional endoscopic sinus surgery in children. J Laryngol Otol. 2009 Jan;123(1):75–79. doi: 10.1017/S0022215108003678. [DOI] [PubMed] [Google Scholar]
- 48.Kim HY, Dhong HJ, Chung SK, Chung YJ, Min JY. Prognostic factors of pediatric endoscopic sinus surgery. Int J Pediatr Otorhinolaryngol. 2005 Nov;69(11):1535–1539. doi: 10.1016/j.ijporl.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Siedek V, Stelter K, Betz CS, Berghaus A, Leunig A. Functional endoscopic sinus surgery--a retrospective analysis of 115 children and adolescents with chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol. 2009 May;73(5):741–745. doi: 10.1016/j.ijporl.2009.01.019. [DOI] [PubMed] [Google Scholar]


