Table 2.
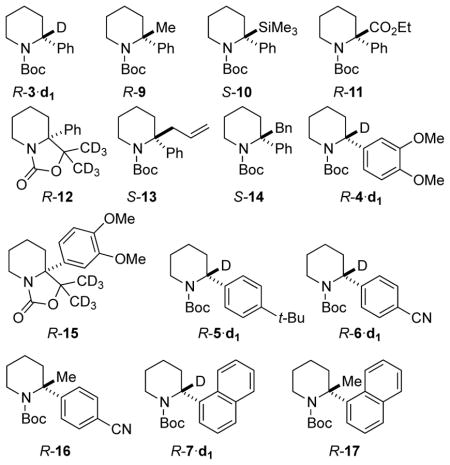
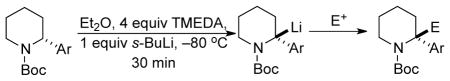
Lithiation-substitution of enantioenriched N-Boc-2-arylpiperidines (see also Figure 1B).
| ||||||
|---|---|---|---|---|---|---|
| Entry | Ar | er (R:S) | E+ | Product | % Yield | er of product |
| 1 | Ph | 96:4 | MeOD | R-3·d1 | 100a | 96:4 |
| 2 | Ph | 96:4 | Me2SO4 | R-9 | 79 | 95:5 |
| 3 | Ph | 96:4 | Me3SiCl | S-10 | 88 | 96:4 |
| 4 | Ph | 96:4 | EtOCOCl | R-11 | 85 | 96:4 |
| 5 | Ph | 96:4 | (CD3)2CO | R-12 | 90 | 95:5 |
| 6 | Ph | 96:4 | Allyl-Brb | S-13 | 66 | 92:8 |
| 7 | Ph | 96:4 | BnBrb | S-14 | 71 | 94:6 |
| 8 | 3,4-(MeO)2-C5H3 | 97:3 | MeOD | R-4·d1 | 100a | 97:3 |
| 9 | 3,4-(MeO)2-C6H3 | 97:3 | (CD3)2CO | R-15 | 93 | 97:3 |
| 10 | 4-(tBu)-C6H4 | 90:10 | MeOD | R-5·d1 | 100a | 90:10 |
| 11 | 4-(NC)-C6H4 | 90:10 | MeOD | R-6·d1 | 100a | 90:10 |
| 12 | 4-(NC)-C6H4 | 90:10 | Me2SO4 | R-16 | 71 | 90:10 |
| 13 | 1-Np | 97:3 | MeOD | R-7·d1 | 100a | 97:3 |
| 14 | 1-Np | 97:3 | Me2SO4 | R-17 | 74 | 93:7 |
% conversion by GC
via zinc and copper-mediated coupling.