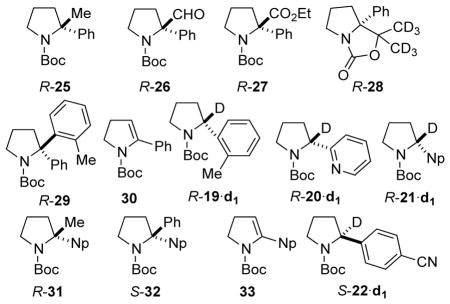
Table 3.
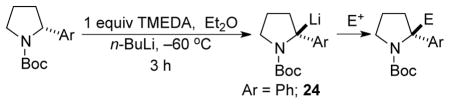
Lithiation-substitution of enantioenriched N-Boc-2-aryl pyrrolidines using indicated conditions, except as noted.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Ar | er (R:S) | E+ | Product | % Yield | er |
| 1 | Ph | 96:4 | Me2SO4 | R-25 | 86a | 94:6 |
| 2 | Ph | 96:4 | DMF | R-26 | 83b (88c) | >99:1 |
| 3 | Ph | 96:4 | EtOCOCl | R-27 | 70b (79c) | 94:6 |
| 4 | Ph | 96:4 | (CD3)2CO | R-28 | 85b (92c) | 94:6 |
| 5 | Ph | 96:4 | 2-bromotoluene | R-29 | 8b,d | 92:8 |
| 6 | Ph | 50:50 | 2-bromotoluene | rac-29 | 12a,d,e | 50:50 |
| 7 | 2-tolyl | 90:10 | MeOD | R-19·d1 | 100c | 90:10 |
| 8 | 2-pyridyl | 90:10 | MeOD | R-20·d1 | 100c | 90:10 |
| 9 | 1-Np | 95:5 | MeOD | R-21·d1 | 100c | 95:5 |
| 10 | 1-Np | 95:5 | Me2SO4 | R-31 | 91a | 95:5 |
| 11 | 1-Np | 95:5 | PhBr | S-32 | <5c,d,e | 95:5 |
| 12 | 4-(NC)-C6H4 | 7:93 | MeOD | S-22·d1 | 100c | 93:7 |
isolated yield,
isolated yield of C2 and C5 substitution products after deprotonation by s-BuLi/TMEDA,
percent conversion by GC after deprotonation using n-BuLi/TMEDA,
via Pd-catalyzed coupling for 48 h at 40 °C,
lithiated in the absence of TMEDA.