Abstract
In mammals, low-density lipoprotein receptor-related protein-2 (LRP2) is an endocytic receptor that binds multiple ligands and is essential for a wide range of physiological processes. To gain new insights into the biology of this complex protein, we have initiated the molecular characterization of the LRP2 homolog from an oviparous species, the chicken (Gallus gallus). The galline LRP2 cDNA encodes a membrane protein of 4658 residues. Overall, the galline and human proteins are 73% identical, indicating that the avian gene has been well conserved over 300 million years. Unexpectedly, LRP2 transcript and protein levels in the kidney of females and estrogen-treated roosters were significantly higher than those in untreated males. The estrogen-responsiveness of avian LRP2 may be related to the dramatic differences in lipoprotein metabolism between mature roosters and laying hens. Newly identified potential estrogen-responsive elements (ERE) in the human and galline LRP2 gene, and additional Sp1 sites present in the promoter of the chicken gene, are compatible with both direct estrogen induction via the classical ligand-induced ERE pathway and the indirect transcription factor crosstalk pathway engaging the Sp1 sites. In agreement with this assumption, estrogen induction of LRP2 was observed not only in primary cultured chicken kidney cells, but also human kidney cell lines. These findings point to novel regulatory features of the LRP2 gene resulting in sex-specific receptor expression.
Abbreviations: Apo, Apolipoprotein; BRCA1, Breast cancer 1; CK-II, Casein kinase-II; DAPI, 4′,6′-diamidino-2′-phenylindole; ERE, Estrogen responsive element; ER, Estrogen receptor; GAPDH, Glycerinaldehyd-3-phosphat-Dehydrogenase; GFP, Green fluorescent protein; gg, Gallus gallus; Gp330, Glycoprotein 330; GSK3, Glycogen synthase kinase-3; GST, Glutathione S-tranferase; HDL, High-density lipoprotein; HEK-293, Human embryonic kidney cells; HRP, Horseradish peroxidase; hs, Homo sapiens; Ig, Immunoglobulin(s); LA-repeat, LDL receptor type A-repeat; LDL, Low-density lipoprotein; LDL-R, Low-density lipoprotein receptor; LRP, Low-densitiy lipoprotein receptor-related protein; Ni-NTA, nickel-nitrilotriacetic acid; ORF, Open reading frame; PKA, Protein kinase A; PKC, Protein kinase C; RAP, Receptor-associated protein; SDS, Sodium dodecyl sulfate; SHBG, Sex hormone binding globulin; Sp, Stimulating protein; SRE, Sterol-responsive element; VLDL, Very low-density lipoprotein; VLDLR, Very low-density lipoprotein receptor
Keywords: LRP2, LDL-R gene family, Sex specificity, Estrogen‐responsiveness, Chicken
Highlights
► First molecular characterization of a non-mammalian LRP2 ► LRP2 is highly conserved from chicken to man. ► Induction by estrogen of LRP2 in‐vivo and in cultured kidney cells of chicken and man ► Identification of estrogen-responsive elements in the galline and human genes
1. Introduction
LRP2, also called megalin or glycoprotein-330 (gp330), was originally identified as the pathogenic autoantigen of Heymann nephritis in rats (Kerjaschki and Farquhar, 1982, 1983). In mammals, this multifunctional cell-surface receptor, the largest member of the low-density lipoprotein receptor (LDL-R) family, is a 600 kDa protein (Nykjaer and Willnow, 2002). LRP2 is a classical endocytic receptor, which is expressed on the apical surface of absorptive epithelia in a number of different tissues, and mainly in glomeruli and cells of the convoluted proximal tubule of the kidney. It binds a wide spectrum of structurally and functionally different ligands (Birn, 2006; Gliemann, 1998; Herz and Strickland, 2001), including vitamins, carrier proteins, lipoproteins, enzymes, hormones, and others (reviewed in (Birn, 2006; Willnow and Nykjaer, 2010)). Importantly, the binding of most ligands is strictly Ca2 +-dependent (Christensen et al., 1992; Hjalm et al., 1996), which is a characteristic property of the LDL receptor family. Various studies have described LRP2 as a carrier for lipoprotein cholesterol and vitamins A and D (Liu et al., 1998; Moestrup and Verroust, 2001). The receptor binds complexes of 25-OH vitamin D3 with vitamin D binding protein (DBP) (Marino et al., 2001; Nykjaer et al., 2001) and of vitamin A with retinol binding protein (RBP) (Christensen et al., 1999). Following internalization, the carrier proteins are degraded in lysosomes, while the vitamins are released into the cytosol to perform their respective functions. This mechanism prevents urinary loss of vitamins in the proximal tubules. Loss of LRP2 expression, e.g. in knockout mice, results in vitamin D deficiency and in bone-calcification defects, underscoring the receptor's prominent role in vitamin homeostasis (Muller et al., 2003; Nykjaer et al., 2001). Accordingly, decreased renal LRP2 expression has been demonstrated in certain diseases characterized by proteinuria (Christensen et al., 2003; Norden et al., 2002; Piwon et al., 2000).
In mammals, LRP2 has been identified as a receptor mediating the uptake of sex hormone binding globulin (SHBG), a carrier protein for androgens and estrogens (Hammes et al., 2005). Steroid hormones act by entering target cells and subsequently associating with nuclear hormone receptors that activate transcription of steroid-responsive genes. LRP2 is expressed in a number of steroid-responsive tissues such as the male and female reproductive organs (Hammes et al., 2005; Zheng et al., 1994), in agreement with its prominent role in steroid hormone function.
LRP2 also plays an important role in the formation of the central nervous system. Homozygous disruption of the corresponding gene leads to malformation of the forebrain and cephalic midline structure, known as holoprosencephaly (Nykjaer and Willnow, 2002; Willnow et al., 1996). Based on its ability to bind apolipoprotein B-100 (Stefansson et al., 1995), apolipoprotein E, and lipoprotein lipase (reviewed in (Christensen and Birn, 2002)), LRP2 has discrete roles in lipoprotein metabolism. Furthermore, LRP2 binds apolipoprotein J (clusterin) with high affinity (Kounnas et al., 1995) and can mediate HDL endocytosis via forming a complex with cubilin and amnionless (Pedersen et al., 2010). Genetic variations in the LRP2 gene influencing lipid metabolism have been described (Mii et al., 2007).
LRP2 homologs have been identified in non-mammalian vertebrates, in which mostly its roles in developmental processes were studied. Yochem et al. characterized an LRP2 homolog, then termed LRP-1, in the nematode Caenorhabditis elegans (C. elegans), and showed essential roles during growth and development (Yochem and Greenwald, 1993; Yochem et al., 1999). The work of Anzenberger at al. provided genetic evidence of LRP2 being present in the larval zebrafish. The protein was reported to be localized in the proximal part of the pronephros (Anzenberger et al., 2006). Christensen at al. showed that Xenopus is expressing a LRP2 homolog in the pronephric kidney (Christensen et al., 2008). To our knowledge, LRP2 homolog(s) have not been reported in avian species to date.
Previous studies in the chicken kidney have shown that this organ, in addition to liver and intestine, synthesizes apoB-100 and apoA-I as constituents of plasma lipoproteins (Blue et al., 1980; Kirchgessner et al., 1987; Tarugi et al., 1998; Walzem et al., 1999). While intestinal and kidney apoB-100 syntheses are not known to be regulated by estrogen (Kirchgessner et al., 1987; Lazier et al., 1994), hepatic apoB-100 synthesis is regulated by the hormone (Capony and Williams, 1980; Chan et al., 1976; Hermann et al., 1997; Luskey et al., 1974; Walzem et al., 1999). In addition, the small apolipoprotein apoVLDL-II is produced in strictly estrogen-dependent fashion exclusively in the liver (Codina-Salada et al., 1983; Walzem et al., 1999; Wiskocil et al., 1980).
In contrast to our knowledge about apolipoprotein regulation by estrogen, information on possible steroid sensitivity of LDL-R family member expression in the chicken is limited to a study by Hummel et al. on the regulation of the chicken LDL receptor by estrogen (Hummel et al., 2003). In an effort to elucidate novel aspects of LRP2 biology, we have now molecularly characterized chicken LRP2 and reveal that LRP2 expression is induced by estrogen in-vivo and in-vitro. Furthermore, we have identified potential estrogen responsive elements (ERE) in the promoter region of the gene, and demonstrate a sex-specific difference in LRP2 levels in the kidney of mature animals.
2. Material and methods
2.1. Animals
Sexually mature, Derco brown (TETRA-SL) laying hens and roosters, as well as 10-week and 10-day old animals, respectively, of both sexes were purchased from Diglas Co. (Feuersbrunn, Austria) and maintained on layer's mash with free access to water and feed under a daily light period of 16 h. Where indicated, roosters were treated by intramuscular injection with 10 mg/kg body weight of 17α-ethinylestradiol (Sigma); stock solution, 40 mg/ml 1,2-Propanediol) either once, or every 24 h for up to 3 times, and sacrificed for tissue and organ retrieval. Freshly fertilized eggs were used and incubated at 37.5 °C and 60–70% humidity. All animal procedures were approved by the Animal Care and Use Committee of the Medical University of Vienna.
2.2. Preparation of genomic DNA and total RNA, cDNA synthesis, PCR, DNA cloning and sequencing
Genomic DNA was isolated from EDTA–treated whole blood using the illustra blood genomicPrep Mini Spin Kit (GE Healthcare) according to the manufacturer's instructions for nucleated blood. Total RNA was extracted using TRI Reagent (MRC, Inc.) following the manufacturer's instructions. Single-stranded cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen) and oligo(dT)18-primer. PCR amplification was performed with a T3000 Thermocycler (Biometra) with a touch-down program using the High Fidelity PCR Enzyme Mix (Fermentas). The amplified products were subjected to 1% agarose gel electrophoresis and stained with ethidium bromide. Subsequently, the PCR product was excised from the gel, and DNA was purified with QIAquick Gel Extraction Kit (Qiagen). The purified product was cloned into the pCR2.1-TOPO vector with the TOPO TA Cloning Kit (Invitrogen), and subsequently transformed into E. coli Top10 cells. The plasmids were isolated using Mini-preparation. Plasmids were sent to Agowa GmbH, Berlin, Germany for sequence analysis.
The following LRP2-specific primers were used: Gallus gallus: forward primer 2, 5′-GGA GTG TTA GCG ATT GGA GGC-3′ and reverse primer 2, 5′-CCT CTT TAA CAA GAT TGG CGG-3′; forward primer 3, 5′-AAA GGC AAA GGA GGC AGG AG-3′ and reverse primer 3, 5′-CCG TAG GAG AAC AGC GCT TG-3′; forward primer 2, 5′-GGA GTG TTA GCG ATT GGA GGC-3′ and reverse primer 4, 5′-CCA CAC TAC CAG CTC CTG TTA-3′. Homo sapiens: forward primer 5, 5′-GAC GCA CGG GCC ATA GTT TGC-3′ and reverse primer 5, 5′-TTT AGG AGG CTG AGG CAG GCG G-3′.
In order to identify potential estrogen responsive elements and for chicken LRP2 sequence verification, we used primers upstream of the designated exon 1 in the genomic sequence found in the galline genomic database (NCBI, gene ID 424168). A new first exon and a large first intron were identified by using the following pimers: forward primer 1, 5′-ATG GGA ACT CGG CAG CAG ACG-3′ and reverse primer 1, 5′-TGA CAG CGA TAC TGG CAG CTC-3′. The obtained mRNA sequence corresponding to chicken LRP2 was deposited in EMBL Nucleotide Sequence Database under the accession HE578280.
2.3. Quantitative real‐time PCR
Quantitative real‐time PCR (qPCR) was performed with the LightCycler 480 system (Roche) using the LightCycler FastStart DNA Master SYBR Green I Kit (Roche). The following primers were used: Gallus gallus: LRP2, forward primer 2 and reverse primer 4; β-actin, forward primer, 5′-AGC TAT GAA CTC CCT GAT GG-3′ and reverse primer, 5′-ATC TCC TTC TGC ATC CTG TC-3′. Homo sapiens: LRP2, forward primer, 5′-GCT GCA GAA AGT CTG GCT GTA-3′ and reverse primer, 5′-TAC TCT CCC AAT GTA TGC GCG G-3′; β-actin, forward primer, 5′-GCG GGA AAT CGT GCG TGA CAT T-3′ and reverse primer, 5′-GAT GGA GTT GAA GGT AGT TTC GTG-3′; apolipoprotein AI (Apo AI), forward primer, 5′-GGC AGC AAG ATG AAC CCC CCC-3′ and reverse primer, 5′-CTG CCT CAG GCC CCT CTG TCT C-3′, and for estrogen receptor α (ERα), forward primer, 5′-ACT ATG CTT CAG GCT ACC A-3′ and reverse primer, 5′-CAA GGC ACT GAC CAT CTG-3′. Diluted cDNA samples were used for all qPCR reactions. Serial dilutions of 10− 1 to 10− 9 of the target PCR products were freshly prepared. All samples were analyzed in duplicates and compared to the serial dilutions serving as internal standard. Chicken and human β-actin mRNA levels were measured as housekeeping genes and used for normalization. Values are expressed in arbitrary units (AU) relative to β-actin.
2.4. Statistical analysis
All values are expressed as means ± SEM of relative mRNA levels. The Student's t-test was employed to explore whether differences in the parameters measured with the qPCR were statistically significant between males and females or between treated and untreated samples. The significance level was set at p < 0.05 (*p < 0.05, **p < 0.01, ***p < 0.001).
2.5. Protein expression and antibody production
A 701 bp chicken LRP2 cDNA fragment encoding the intracellular domain (Fig. 3A) with a C-terminal 6xHis-tag was generated by RT-PCR using the following oligonucleotides: forward, 5′-GAA TTC GGA GTG TTA GCG ATT GGA GGC-3′ (EcoRI restriction site in boldface) and reverse, 5′-GCG GCC GCT TAA TGA TGA TGA TGA TGA TGA TGC TCT TTA ACA AGA TTG GCG GTG-3′ (NotI restriction site in boldface, and 6xHis-tag sequence in italics). The fragment was cloned into the pGEX-5X-1 expression vector to provide an N-terminal GST-tag. The cloned ggGST-LRP2-His-construct was expressed in BL21 cells (Invitrogen) and purified using Ni-NTA beads technology from QIAgen. Antiserum against recombinant ggLRP2 was raised in adult female New Zealand White rabbits by injections of antigen, 200 mg each, as described previously (Hummel et al., 2003). Mouse anti-glutathione S-transferase (GST) antibody was purchased from BD Bioscience and used at a dilution of 1:2000.
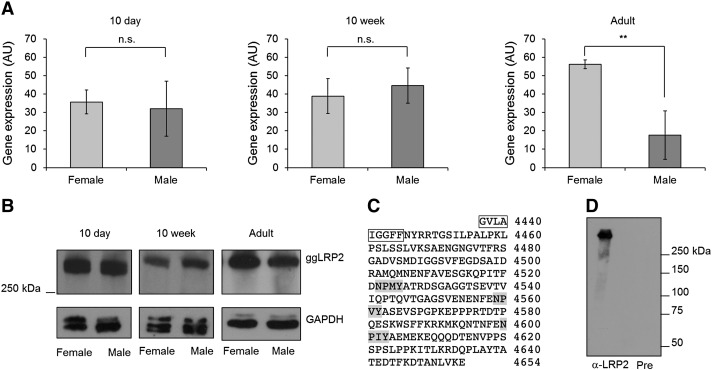
Fig. 3.
LRP2 expression in kidneys of young, immature, and adult animals. A: Quantitative real-time PCR was performed as described in Material and methods. Each set of data shown is representative of three individual animals. Kidneys of male and female animals aged 10 days, 10 weeks, or at least 25 weeks were used for cDNA synthesis and subsequent quantitative RT-PCR experiments. All values are expressed as means ± SEM of mRNA levels relative to those of β-actin; all experiments were performed in triplicate. Data were analyzed by Student's t-test (**, p < 0.01). B: Triton X-100 membrane protein extracts of female and male kidneys of 10 day, 10 week (30 μg protein/lane), and adult (60 μg protein/lane) animals were subjected to SDS-PAGE under non-reducing conditions and subsequent Western blot analysis as described in Material and methods. As loading controls, GAPDH levels were monitored. (MW.: LRP2 ~ 600 kDa, GAPDH ~ 37 kDa). C: protein sequence of the ggLRP2 fragment (residues 4437–4654) used for raising rabbit anti-LRP2 antiserum. The part of the predicted transmembrane domain (boxed) and the three NPXY (Asn-Pro-Xxx-Tyr) internalization motifs (shaded) are indicated. D: Western blot demonstrating the reactivity of the antiserum (used at 1:1000 dilution) against a membrane extract of kidney, the main expression site of LRP2; as mammalian LRP2 (Saito et al., 1994), the chicken homolog migrates at approximately 600 kDa (lane 1). Lane 2, incubation with pre-immune serum (1:1000). Triton X-100 extracts prepared from laying hen kidney (20 μg protein/lane) were subjected to 6% SDS-PAGE under non-reducing conditions and processed for immunoblotting.
2.6. Preparation of membrane protein extracts and cell lysates
Membrane protein fractions were prepared from fresh chicken tissues as described (Stifani et al., 1988), except that the extraction buffer contained 5% Triton X-100. The clear supernatant, designated the membrane extract, was either used immediately or quickfrozen in liquid nitrogen and stored at − 80 °C until use. For cell lysis, monolayers were washed three times with phosphate-buffered saline (PBS), scraped from the dishes with a rubber policeman, centrifuged at 2000 ×g for 5 min, and the cell pellet was solubilized by addition of buffer B (200 mM Tris-maleate, pH 6.5, 2 mM CaCl2, complete protease inhibitor cocktail (Roche), and 1.4% Triton X-100). The cell extracts were centrifuged at 300,000 ×g (Beckman TLA 100.3 rotor) for 40 min at 4 °C and the pellets discarded. The supernatants were subjected to one-dimensional SDS-PAGE as described below.
2.7. SDS-polyacrylamide gel electrophoresis and immunoblotting
For Western blotting, protein concentrations were determined by the method of Bradford (Bio-Rad), aliquots of extracts were subjected to 6% or 12% SDS-PAGE under non-reducing conditions, and the separated proteins electrophoretically transferred to nitrocellulose membranes (Hybond-C Extra; Amersham Biosciences). The amounts loaded were monitored by Ponceau S staining of the membranes. Nonspecific binding sites were blocked with TBS (20 mM Tris–HCl, pH 7.4, and 137 mM NaCl) containing 5% (w/v) nonfat dry milk and 0.1% Tween-20 (blocking buffer) for 1 h at room temperature. GgLRP2 was detected with rabbit anti-ggLRP2 antiserum (1:1000) followed by incubation with HRP-conjugated goat anti-rabbit IgG (1:50,000, Sigma-Aldrich) and development with the enhanced chemiluminescence protocol (Pierce). For additional analyses of the loading controls (GAPDH), a monoclonal mouse anti-panGAPDH antibody (1:20,000, Sigma-Aldrich) was used in combination with an HRP-conjugated goat anti-mouse IgG (1:20,000, Jackson Immuno Laboratories, Inc.), and the blots were developed as described above to detect the ~ 37 kDa signal.
2.8. Immuno-histo- and -cytochemistry
Freshly isolated tissues were fixed overnight in 4% paraformaldehyde and embedded in paraffin using an Excelsior embedding machine. Sections of 5 μm were cut on a Microm HM335E microtome, fixed on Polysine slides (Menzel-Glaeser) and dried overnight at 37 °C. The slices were deparaffinized in xylol exchange medium XEM-200 (Vogel, Giessen, Germany) by gentle shaking for 20 min. For rehydration, the tissues were consecutively washed in 100%, 90%, 70%, 50%, and 30% ethanol. Primary kidney cells were grown on chamber slides (Nunc, Inc.) and fixed by incubation with ice-cold acetone:methanol (1:1, vol:vol) for 10 min. Cells were rinsed 3 × 5 min with PBS.
Nonspecific binding of antibodies was inhibited by blocking with PBS containing 1% BSA and 3% inactivated goat serum for 1 h at RT. The sections were incubated overnight at 4 °C with the appropriate antibodies in blocking solution. For immunofluorescence, the sections were rinsed 3 × 5 min in PBS and incubated with goat α-rabbit fluorescence-labeled secondary antibodies (Alexa Fluor 488 conjugated, 1:1000) for 1 h. Counterstaining of cell nuclei was performed with DAPI (1:1000). Specimens were mounted in fluorescent mounting media (DAKO) and analyzed by fluorescence microscopy (Axiovert 135, Zeiss). For biotin staining, the sections were rinsed 3 × 5 min in PBS and incubated with biotinylated goat α-rabbit secondary antibody (1:500 dilution, Sigma) for 1 h. After rinsing 3 × 5 min in PBS, the slides were incubated with Streptavidin Peroxidase Polymer (1:200, Sigma) for 1 h. For the color reaction, the sections were incubated with AEC + Substrate-Chromogen (ready-to-use solution, DAKO). The specimens were mounted in Glycergel Mounting Medium (DAKO) and analyzed by light microscopy (Axiovert 10, Zeiss).
2.9. Cell culture
The cell line HEK-293 (human embryonic kidney cells) was purchased from Cell Lines Service (Eppelheim, Germany). These cells were originally isolated from primary human embryo kidney cells transformed by sheared adenovirus 5 DNA (Graham et al., 1977). HEK-293 cells were cultivated in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 Ham. The medium contained 10% fetal calf serum (FCS), 2 mM l-glutamine, 0.1 mg/ml streptomycin, and 1 00U/ml penicillin, and the cells were cultured in a humidified 95% air/5% CO2 incubator. Experiments were performed in phenol-free media with 10% serum with or without addition of 17α-ethinylestradiol as described below.
Primary chicken kidney epithelial cells were isolated from four kidneys pooled from 1 to 3 day old animals of undetermined sex as described previously (Ogburn et al., 1998). Briefly, kidneys were removed and thoroughly rinsed in PBS. The tissue was then minced into smaller fragments and incubated in an enzyme solution containing 1 mg/ml type II collagenase (Sigma) in PBS for 30 min at 37 °C. After collagenase treatment, cells were filtered through a 70 μm nylon mesh, washed twice with PBS, and resuspended in culture medium. Cells were grown in Quantum epithelial medium (PAA, Linz, Austria) supplied with 100 U/ml penicillin in a humidified 95% air/5% CO2 incubator.
Prior to mRNA extraction or protein isolation, the cells were grown to reach 60–70% confluency. Where indicated, cells were treated by addition to the medium of 17α-ethinylestradiol dissolved in ethanol to a final concentration of 50 nM or with vehicle alone.
2.10. Reporter constructs, transfections and luciferase assays
A 905 bp fragment containing the ERE and its flanking Sp1 sites was amplified using the forward primer 5′-ATA CGC GTA AAG GCA AAG GAG GCA GG A-3′ and the reverse pimer 5′-ATC TCG AGT GGG CAG ACC TGC ATT AC-3′ (MluI and XhoI restriction sites in boldface). This sequence was inserted upstream of the promoter-luc + transcriptional unit of the pGL3-Promoter Vector to determine the functionality of the putative enhancer elements. The empty pGL3-Promoter Vector, the pGL3-Control Vector, the pRL-TK (Promega) and the pEGFP-N1 (Clontech) were used as controls.
HEK-293 cells were grown to confluency in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 Ham. The medium contained 10% fetal calf serum (FCS), 2 mM l-glutamine, 0.1 mg/ml streptomycin, and 100 U/ml penicillin, and the cells were cultured in a humidified 95% air/5% CO2 incubator. Experiments were performed in phenol red- and serum-free medium.
Transient transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. HEK-293 cells were plated on 12-well plates and transfected with 1.6 μg of pGL3 vectors or a GFP expression vector, and co-transfected with 0.16 μg of pRL-TK vector (internal control). After 6 h the medium was replaced and cells were treated with vehicle alone or with a final concentration of 50 nM estrogen in phenol red- and serum-free medium. After 24 h, the cells were harvested in passive lysis buffer (Promega) and analyzed using the Dual-Luciferase Reporter Assay System (Promega). Luminescence was measured using a Victor3V (PerkinElmer) reader and reported as a ratio of firefly luciferase/Renilla luciferase.
3. Results
3.1. Nucleotide sequence analysis reveals alternative first exon
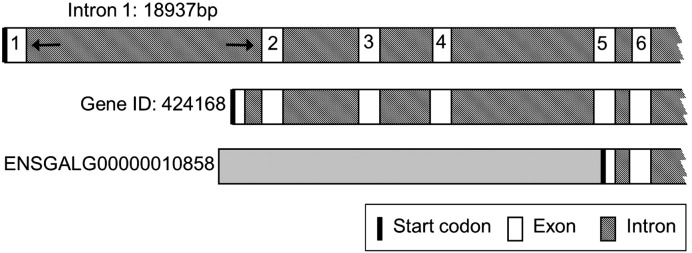
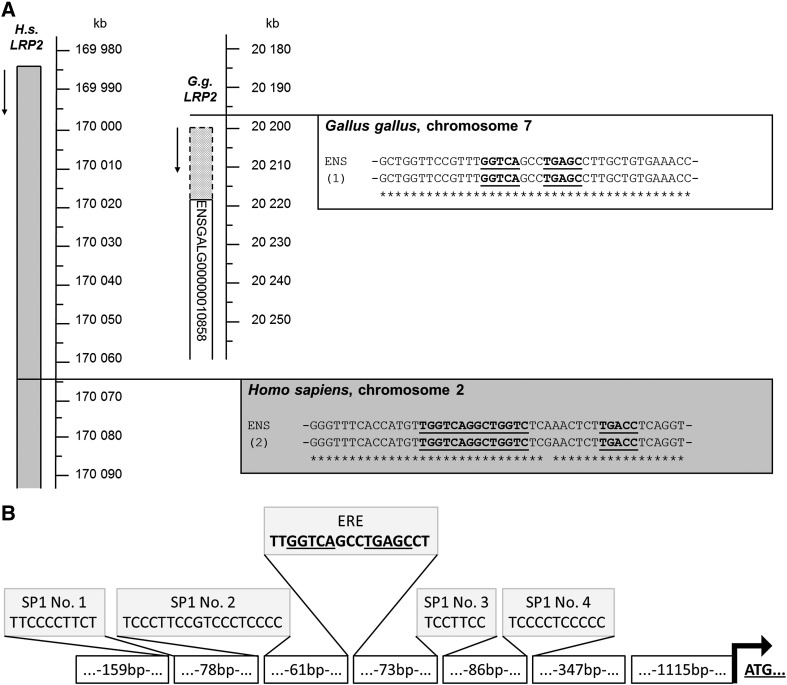
We identified transcripts of 13,935 bp (ENSGALT00000017651) and 13,938 bp (ENSGALT00000020276), respectively, in the Ensembl chicken genome database, and a 15,165 bp transcript with the accession number XM_422014 (NCBI). For chicken LRP2 sequence verification, we used primers upstream of the designated exon 1 of gene ID 424168 (NCBI) as described in detail in Material and methods. For analysis of the 5′ region and for identification of potential estrogen responsive elements, total RNA from chicken kidney was reverse-transcribed into cDNA, and PCR was performed using forward and reverse primer 1 (see Material and methods). The obtained 971 bp sequence aligned with the sequence of contig 19.532 found on galline chromosome 7, but the predicted sequences in the region of the first exon and intron were different from the sequences we obtained. We identified a 95 bp first exon and a 18,937 bp long first intron, resulting in the longest open reading frame (ORF) by comparison with the predicted sequence (Fig. 1). The two designated LRP2 gene sequences, ENSGALG00000010858 (Ensembl chicken genome database) and 424168 (NCBI), lack parts of the 5′-region as detailed in Fig. 1. The newly revealed transcript spans 13,977 bp, which encodes a protein of 4658 amino acids. There is 73.0% identity between the human and avian LRP2 protein sequences. The mature protein has a calculated molecular mass of 521.39 kDa. Chicken (Gallus gallus; gg) LRP2 is a predicted type I transmembrane protein consisting of a 31 amino acid signal peptide, an extracellular region of 4391 amino acids, and a single transmembrane domain of 23 amino acids. An intracellular C-terminal region of 213 residues contains three (FX)NPXY signals for coated pit-mediated internalization, similar to the motif (VD)NKNY in the cytoplasmic tail of human LRP2. As the extracellular region of its mammalian counterparts, ggLRP2 contains four clusters of cysteine-rich LA-repeats, characteristic of the LDLR superfamily of receptors (Hjalm et al., 1996). Each of the clusters are comprised of a specific number of LA-repeats and represent a putative ligand binding site for a diverse group of ligands; for instance, the second cluster binds ApoE-β-VLDL, lactoferrin, aprotinin, lipoprotein lipase, and receptor-associated protein (RAP) (Dolmer and Gettins, 2006; Orlando et al., 1997).
Fig. 1.
Partial genomic organization of the chicken LRP2 locus on gg chromosome 7. Top, scheme representing contig 19.532 on chicken chromosome 7. The newly identified first intron (intron 1, 18,937 bp) and exon 1 (95 bp) are indicated. The resulting transcript is predicted to yield an open reading frame encoding a 4658‐residue protein. As indicated in the two bottom representations, the two previously reported LRP2 gene sequences, ENSGALG00000010858 (Ensembl chicken genome database) and 424168 (NCBI), have multiple sequence differences. As a consequence, the predicted transcript of the NCBI gene sequence lacks parts of the first exon, resulting in a shorter open reading frame (NCBI XM_422014, 4605 aa), whereas ENSGALG00000010858-derived transcripts ENSGALT00000017651 and ENSGALT00000020276 predict the start codon at the indicated position, and yield proteins of 4644 and 4645 amino acids, respectively.
3.2. Analysis of LRP2 transcript levels and protein localization
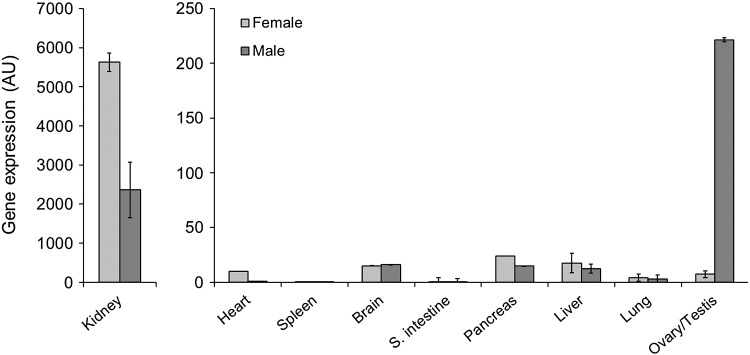
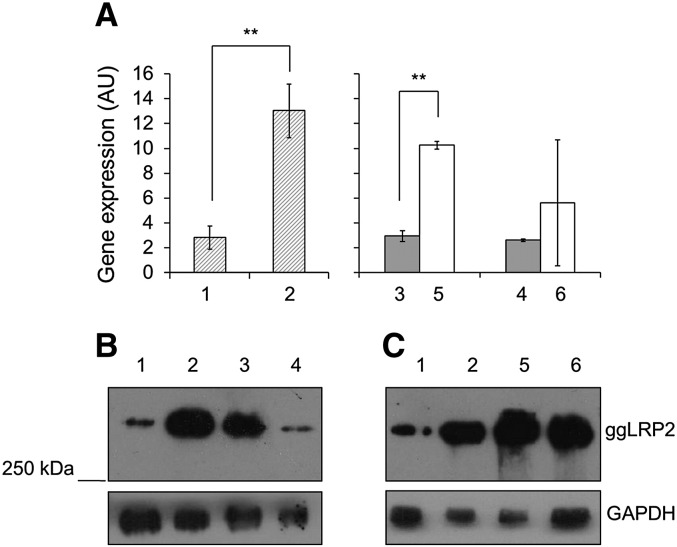
To gain insight into the biology of ggLRP2, we first determined the tissue expression pattern of chicken LRP2 at the transcript level. As shown in Fig. 2, among the tissues analyzed by quantitative real-time PCR, the highest expression was found in kidney, where the level is up to 400 times higher than in all other tissues. Interestingly, we observed a significantly higher level of LRP2 transcript in the kidneys of hens than of roosters, and in testes, the level was 30 times higher than in the ovary, indicating that certain chicken tissues show sex-dependent differences in LRP2. To substantiate this finding, we analyzed the expression of LRP2 at both the transcript and protein levels in 10-day old chicks, 10-week old immature, and adult animals (> 25 week old). To this end, we first raised a rabbit antiserum against chicken LRP2. A ggLRP2 fragment corresponding to amino acid sequences 4437 to 4654 (Fig. 3C) was produced recombinantly, expressed as a His6-tagged GST-fusion protein in BL21 cells, purified, and used to immunize rabbits. The resulting antiserum, directed against the C-terminal region of LRP2, recognized a protein of about 600 kDa in chicken kidney, whereas the pre-immune rabbit serum did not show any reactivity (Fig. 3D). We used this antiserum to analyze in greater detail the expression of ggLRP2 in kidneys by Western blotting, in parallel to measurements of transcript levels. As shown in Fig. 3B, there was significantly more LRP2 protein in kidneys of sexually mature hens than in those from roosters, in good agreement with an observed threefold higher ggLRP2 transcript level in the kidneys of females than in males (Fig. 3A). In kidneys of ten-day as well as ten-week old animals, no significant differences between the sexes in LRP2 expression were observed at both the transcript and the protein level.
Fig. 2.
Tissue distribution and levels of chicken LRP2 transcripts. Quantitative real‐time PCR was performed with the indicated tissues from laying hens and mature roosters. Chicken β-actin mRNA levels were measured as housekeeping gene and used for normalization. Values are expressed as arbitrary units (AU) relative to those for chicken β-actin. All values are expressed as means ± SEM of relative mRNA levels from experiments performed in triplicate.
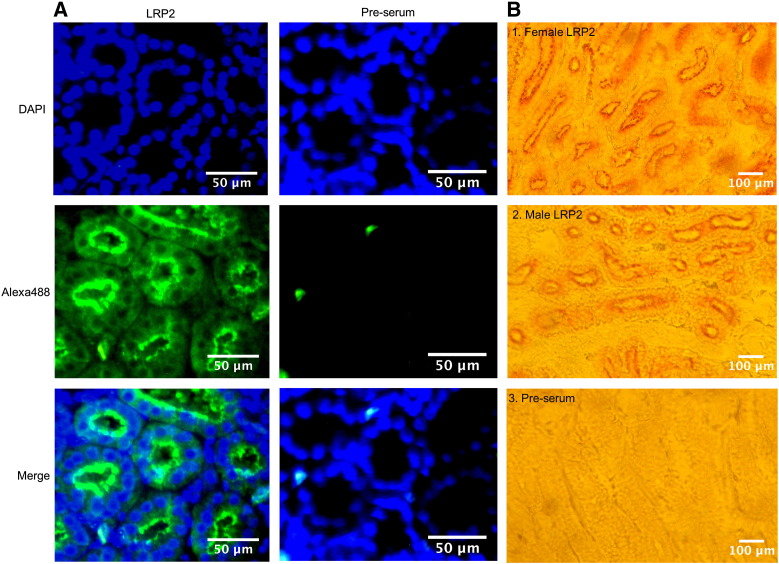
Next, immunoflourescence staining of kidney sections of laying hens and roosters with our anti-chicken LRP2 antibody revealed that LRP2 is predominantly localized to the apical surface of proximal tubules (Fig. 4A), clearly seen in the merged image (lefthand bottom photograph). This localization was confirmed by staining with the anti-chicken LRP2 antiserum followed by biotinylated secondary antibody and detection by streptavidin-HRP-catalyzed color reaction (Fig. 4B). No staining by either method was observed when sections were incubated with pre-immune serum (Fig. 4). Furthermore, the data of Fig. 4B show that immunohistochemistry also reveals higher levels of LRP2 in female than in male kidney, and that the localization of the receptor is identical in the kidneys of both sexes (Fig. 4B, panels 1 vs. 2).
Fig. 4.
Localization of LRP2 in chicken kidney. A: Immunofluorescence staining (see Material and methods) of paraffin sections of female kidney with anti-chicken LRP2 antiserum (1:100) or pre-immune serum (1:100), and nuclear staining with DAPI (magnification, 40 ×). B: Paraffin sections of laying hen (B1. and B3.) and rooster (B2.) kidneys were incubated with anti-chicken LRP2 antiserum (1:1000) or with preimmune serum (1:1000). After the primary antibody, biotinylated goat α-rabbit secondary antibody (1:500 dilution) was used, and slides were incubated with Streptavidin Peroxidase Polymer (1:200). AEC + Substrate-Chromogen was used to detect LRP2 by color reaction. Magnification, 20 ×.
3.3. Estrogen regulates LRP2 expression in the kidney and in cultured cells
To directly investigate the effects of estrogen, we treated mature roosters with estrogen, a treatment shown to induce dramatic metabolic changes in these animals (Chan et al., 1976; Kirchgessner et al., 1987; Lazier et al., 1994; Luskey et al., 1974; Walzem et al., 1999). The roosters were treated either with vehicle alone or with one to three doses of 10 mg estrogen/kg bodyweight each, administered every 24 h. As described in the legend to Fig. 5, control and treated animals were sacrificed at different time points and subsequently quantitative real-time PCR was performed to determine renal LRP2 transcript levels (Fig. 5A). 24 h after a single dose of estrogen, the LRP2 mRNA level increased more than 4-fold (bar 2 vs. 1); at 48 h after the single dose (bar 3), the level had returned to that in untreated roosters, and remained low for another 24 h (bar 4). However, multiple estrogen administration led to induction of LRP2 transcription that persisted over the given timeframe, albeit apparently blunted by consecutive treatments (compare bars 2, 5, and 6). To evaluate the effects of estrogen on renal levels of LRP2 protein, we performed Western blot analyses (Figs. 5B and C). As at the transcript level, a single stimulation with estrogen revealed a rapid increase of LRP2 protein after 24 h. The return to baseline was attenuated compared with that of the transcript levels (Fig. 5B, lanes 1–4) after the single dose; however, multiple doses led to elevated levels of protein that were more persistent than the increase in transcript Fig. 5C). These data indicate strong effects of estrogen on transcription of the LRP2 gene, in turn leading to increased levels of the receptor protein, which at least in the kidney appears to turn over rather slowly.
Fig. 5.
Effects of estrogen on LRP2 expression in rooster kidney. Samples for qPCR (A) or Western blotting (B and C) were prepared as described in Material and methods from kidneys of roosters treated with or without estrogen (each dose consisted of vehicle alone or 10 mg/kg bodyweight) as follows: 1, vehicle only; 2, a single dose at 0 h, sacrificed at 24 h; 3, a single dose at 0 h, sacrificed at 48 h; 4, a single dose at 0 h, sacrificed at 72 h; 5, 1 dose each at 0 and 24 h, sacrificed at 48 h; and 6, 1 dose each at 0, 24, and 48 h, sacrificed at 72 h. A: Quantitative real-time PCR of LRP2 mRNA. All values are expressed as means ± SEM of mRNA levels relative to those of β-actin from experiments performed in triplicate. Data were analyzed by using the Student's t-test (** p < 0.01). B and C: per lane, 20 μg protein of the indicated Triton X-100 kidney membrane extracts were subjected to Western blot analysis following SDS-PAGE under non-reducing conditions with anti-chicken LRP2 antiserum (1:1000 dilution) as described in Material and methods. The anti-panGAPDH antibody (1:20,000) was used to detect the product of the housekeeping gene. (MW.: LRP2 ~ 600 kDa, GAPDH ~ 37 kDa).
Next, we investigated the molecular basis for the sex-dependent difference of LRP2 expression. We searched for estrogen-responsive elements (ERE) in the gene for LRP2. By analysis in silico, we were able to identify potential ERE in both the galline (on chromosome 7) and human (on chromosome 2) LRP2 genes (Fig. 6). The chicken ERE, 5′-TT GGTCA GCC TGAGC CT-3′, is a palindromic sequence with a single mismatch (in bold). Very similar elements can be found in the promoter regions of the gene for chicken vitellogenin A2 (5′-GT CCA AAG TCAGGTCA CAG TGAACCTGATCAAAGTT-3′), for chicken apo very low‐density lipoprotein II (apoVLDL-II) (− 221: 5′-GGGCT CAG TGACC-3′, − 178: 5′-GGTCA GAC TGACC-3′), and chicken ovalbumin (− 47/− 43: 5′-TG GGTCA-3′, a “half ERE”) (Klinge, 2001). In the human LRP2 gene we found the ERE sequence, 5′-T GGTCA GGC TGGTC TCG AAC TCT TGACCTC-3′, which is identical to the element existing in the human breast and ovarian cancer susceptibility gene BRCA1. We confirmed these findings by sequence analysis, which located the ERE of the ggLRP2 gene 1659 bp upstream of the start codon (Fig. 6A); the ERE of the human LRP2 gene is situated in intron 38 of the gene sequence (Fig. 6A). Furthermore, we identified four Sp1 sites flanking the ERE in the galline LRP2 promoter region, an important finding in regards to the possible mechanism underlying the estrogen induction of this gene (see Discussion). A schematic overview of the genomic organization is shown in Fig. 6B.
Fig. 6.
A: Location of estrogen-responsive elements (ERE) in the LRP2 genes of Gallus gallus and Homo sapiens. The magnified regions show alignments of the respective published Ensembl gene sequence (ENS) with the sequences we obtained for G. gallus (1; upper box) and H. sapiens (2; shaded lower box). The presumptive EREs are underlined and bold. In chicken, the ERE sequence is located in the promoter region of LRP2, 1659 bases upstream of the start codon we identified. The ERE in the human LRP2 gene is located in intron 38. B: Schematic representation of the localization of the four Sp1 sites relative to the ERE and the start codon in the promoter region of galline LRP2.
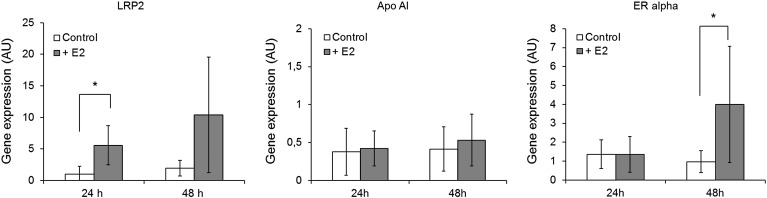
These findings prompted us to attempt to shed light on LRP2 regulation by estrogen in human and galline cells. The effect of estrogen on human LRP2 expression was studied in an established cell line derived from human embryonic kidney (HEK-293). In this cell line, quantitative real-time PCR demonstrated an increase in LRP2 transcription relative to controls upon exposure to 50 nM estrogen. A significant induction was observed after 24 h, (Fig. 7; p < 0.05). The mRNA levels of apolipoprotein (Apo) AI did not respond to estrogen treatment under these conditions, in agreement with published data (Harnish et al., 1998; Staels et al., 1989). As a positive control, the transcription of ERα, which is known positively regulated by hormone treatment, was analyzed (Hofmeister et al., 2012; Kummer et al., 2011; Treilleux et al., 1997). We indeed observed an approximately 5-fold transcript induction after 48 h.
Fig. 7.
Estrogen treatment of HEK-293 cells is affecting gene expression. Quantitative real-time PCR was performed as described in Experimental Procedures. Each set of data shown is representative of three independent experiments. The cells were treated with vehicle alone (open bars) or with 50 nM estrogen (gray bars) and harvested for analysis after the indicated times. The mRNA levels of LRP2, Apo AI, and ERα were analyzed. All values are expressed as means ± SEM of mRNA levels relative to those of β-actin; the experiments were performed in triplicate. Data were analyzed by Student's t-test (*, p < 0.05).
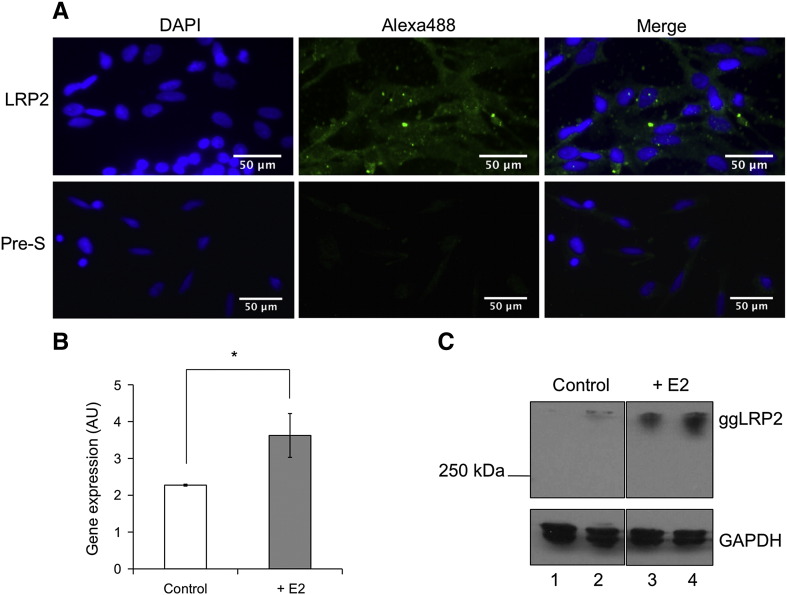
Finally, we isolated primary chicken kidney epithelial cells from the organs of 1–3 day old chicks of undetermined sex. By immunfluorescence staining (Fig. 8A) of primary cells with anti-chicken LRP2 antibody, we demonstrated that LRP2 is indeed expressed in these cells. To study the estrogen effects on galline LRP2 in the cultured kidney cells, we treated the cells with estrogen for 24 h. Quantitative real-time PCR was performed in order to determine the relative mRNA levels before and after estrogen treatment. As shown in Fig. 8B, a significant increase in the transcription of LRP2 mRNA was noted after 24 h. As in Figs. 3 and 5, the estrogen effects on transcription correlate well with those at the protein level observed by Western blotting of lysates of primary chicken kidney epithelial cells (Fig. 8C).
Fig. 8.
Localization of LRP2 in primary chicken kidney epithelial cells and induction by estrogen. The cells were isolated and prepared for immunocytochemistry with anti-chicken LRP2 antiserum or preimmune serum (Pre-S) as described in Material and methods. A: Immunofluorescence (magnification, 40 ×). B: Quantitative real-time PCR of LRP2 mRNA in primary chicken kidney epithelial cells. Cells were treated with 50 nM estrogen and harvested after 24 h. All values are expressed as means ± SEM of mRNA levels relative to those of β-actin; experiments were performed in triplicate. Data were analyzed by Student's t-test (*, p < 0.05). C: Determination of LRP2 protein levels in lysates of primary chicken kidney epithelial cells treated (lanes 2 and 3) or not treated for 24 h (lanes 1 and 2) with 50 nM estrogen. 50 μg of protein/lane was separated by 6% (or 12%, respectively) SDS-PAGE under non-reducing conditions and processed for Western blot analysis with rabbit anti-ggLRP2 antiserum (1:1000). The anti-panGAPDH antibody (1:20,000) was used to detect the product of the housekeeping gene.
4. Discussion
LRP2 (also termed gp330 or megalin) is the largest known member of the low-density lipoprotein receptor (LDLR) family and arguably possesses the broadest ligand spectrum of these membrane proteins. Although our knowledge about functions and regulation of mammalian LRP2s is extensive, insights into the properties of this receptor in other vertebrates may reveal important additional biological details. Thus, the current studies were focused on the completion of the molecular characterization, on the elucidation of regulatory features, and on gaining insights into possible biological roles of the homolog of the giant membrane protein in the chicken. Careful analysis of the ggLRP2 locus on chromosome 7 revealed that published reports about genomic structure and transcript sequence were largely correct. However, our inspection of and experiments to re-evaluate the sequence information revealed additional interesting details (Fig. 1). An additional 95 bp exon, separated by a newly defined 18,937 bp intron from the hitherto presumed first exon, was demonstrated by reverse transcription PCR, resulting in a transcript of 13,977 bp specifying a 4658-residue protein. GgLRP2 shows all of the hallmark structural elements and the domain organization typical of this endocytic receptor, including three (FX)NPXY internalization motifs in the 213-residue cytoplasmic domain and four clusters of ligand-binding repeats in the large extracellular portion (Saito et al., 1994). Human LRP2 shows high endocytic activity mediated by tyrosine-based endocytic motifs within the cytoplasmatic domain, which also harbors several putative phosphorylation motifs corresponding to protein kinase C (PKC), protein kinase A (PKA), casein kinase-II (CK-II) and glycogen synthase kinase-3 (GSK3) phosphorylation sites (Marzolo and Farfan, 2011). All of these motifs are present in the chicken protein. Yuseff at al. have shown that LRP2 is constitutively phosphorylated by GSK3 at a PPPSP motif enclosed in a proline-rich motif of the cytoplasmic tail (Yuseff et al., 2007), exactly as in the chicken LRP2's tail. Besides the two proline-rich motifs, a PDZ-binding motif has been implicated in the interaction of LRP2 with ligands (Marzolo and Farfan, 2011). Again, both the PPPSP motif and PDZ-binding motif are conserved in LRP2 sequences from different species including man, rodents, and chicken (Marzolo and Farfan, 2011). At the protein level, the sequence identity of galline LRP2 with known mammalian LRP2 is 70–73%, with an identity between LRP2 from, e.g., man and rat of 77%. The observed high degree of conservation of the gene for the extremely large single-chain protein LRP2 from avians to mammals further supports roles for the receptor that are common in a wide range of species despite different physiological settings.
The analogies between LRP2 from mammals and the chicken extend to the tissue distribution pattern, in that the kidney is the major site of expression, with high levels also observed in testes (Fig. 2). The renal receptor is localized to the apical aspect of the epithelial cells lining the proximal convoluted tubules (Fig. 4A), where it likely mediates the resorption of certain proteins from the glomerular ultrafiltrate, as has been demonstrated in normal as well as genetically modified mice (Leheste et al., 2003; Vegt et al., 2011). Quite unexpectedly and to date not observed in any other species, qPCR experiments suggested that LRP2 is expressed at significantly higher levels in the kidneys of mature hens than of roosters (Fig. 3C). This important observation was substantiated by immunological studies with a newly generated rabbit-anti ggLRP2 antiserum (Fig. 3D), which was also used to demonstrate that the receptor's apical localization in the proximal tubular epithelium was unchanged at the higher levels found in female kidneys (Fig. 4B).
Since the observation of an apparent sex difference in LRP2 levels is a novel aspect that may or may not be typical of or limited to the chicken, several experiments to characterize the details of this property were performed. Estrogen is known to have dramatic effects on the expression of genes in the chicken, particularly of those involved in metabolic pathways related and/or essential to the reproductive effort of the hen, i.e., oocyte development and egg-laying (Schneider, 2007). The body of investigations on estrogen's effects in the chicken has been performed by treating mature roosters with pharmacological doses of the hormone (i.e., 17a-ethinylestradiol or 17b-estradiol), whereby a “female” program is rapidly switched on. This effect is closely related to the pivotal function of the liver in synthesizing yolk precursor molecules such as very low‐density lipoprotein (VLDL) particles for uptake into growing oocytes (Schneider, 2009). There appears to be little difference in the efficacy of gene induction by acute hormonal stimulus of roosters versus that leading to elevated levels by the chronic estrogen status of laying hens. While both acutely and chronically elevated estrogens raise yolk precursor protein levels, studies on apolipoprotein (apo)-VLDL-II, apoB, and vitellogenin, 3 major molecules destined for oocyte uptake, have uncovered subtle differences in the induction of these genes (Bergink and Wallace, 1974; Jost et al., 1986; Kirchgessner et al., 1987; Noteborn et al., 1986; Wang and Williams, 1983; Wiskocil et al., 1980). For instance, Bergink and Wallace (1974) observed that upon an initial estrogen administration to roosters the hepatic vitellogenin gene is induced after a considerable lag time and more slowly than following a second injection, which causes an immediate rise and higher final levels of transcript and protein. Similarly, Kirchgessner et al. (1987) found that the induction in-vivo of apoB mRNA by estrogen shows no lag, in contrast to that of apo-VLDL-II. The consequence of priming on the estrogen induction of chicken hepatic genes has been proposed to be based on a “memory” effect, which may or may not be displayed by the gene for apo-VLDL-II (Noteborn et al., 1986; Wiskocil et al., 1980).
While extensive studies have clearly shown that the prime target tissue for estrogens in the mature hen is primarily the liver, certain genes are, at least to some extent, estrogen-responsive in the kidney. For instance, apo-VLDL-II and apoB are major constituents of VLDL particles, and their hepatic expression in embryos is enhanced by estrogen, whereas in the embryonic kidney this is the case only for apo-VLDL-II (Lazier et al., 1994). In the adult hen, the two genes remain estrogen-responsive in the liver, but despite demonstrated synthesis of VLDL particles in the kidney (Walzem et al., 1999), the estrogen-responsiveness of renal apo-VLDL-II is significantly lower, whereas apoB is unresponsive in both intestine and kidney (Kirchgessner et al., 1987). Here we observed that the induction of LRP2 in the kidney at both the transcript and protein level is massive and rapid, as it occurs within 24 h, and the return to baseline appears to be attenuated by repeated hormone administration (see Fig. 5 for details), possibly via the above mentioned „memory“ effect. Furthermore, the observation of elevated LRP2 levels in kidneys in females is corroborated by the fact that LRP2 expression in the kidneys of mature roosters is induced by administration of estrogen (Fig. 5), compatible with a direct effect of the hormone. Alternatively, the estrogen effect in-vivo might be an indirect consequence of physiological changes due to the dramatic metabolic effects of estrogen administration (Chan et al., 1976; Kirchgessner et al., 1987; Lazier et al., 1994; Luskey et al., 1974; Walzem et al., 1999). This possibility seems unlikely, however, as induction by estrogen of LRP2, at both the transcript and protein levels, was also observed in cultured primary chicken renal epithelial cells (Fig. 8) and incidentally also in two established human kidney cell lines (Fig. 7). Therefore, the data suggest that we have identified the galline LRP2 gene as being estrogen-responsive in the kidney, and indicate that the induction kinetics in this organ are similar, if not identical, to those of hepatic estrogen-regulated genes. One consequence of the elevated estrogen levels of the laying hen is the newly revealed sexual dimorphism of renal LRP2.
The molecular basis and biological significance for estrogen action on any gene in the avian kidney are unknown, and thus the identification of candidate sequences for estrogen-responsive elements (ERE) not only in the promoter of the galline, but also of the human LRP2 gene (Fig. 6) provides initial insight into possible mechanisms for the hormonal regulation of LRP2. In considering the sequence of these presumptive ERE, it is known that they can deviate substantially from the consensus sequence (O'Lone et al., 2004). To our knowledge, detailed studies on the regulation by estrogen of genes specifying LDLR family members have been performed only for the human LDLR itself (Bruning et al., 2003; Li et al., 2001). The published data suggest that estrogen activation of transcription of the LDLR gene is likely effected by ligand-dependent interaction of estrogen receptor α and the transcription factor, Sp1 (stimulating protein-1). In fact, an estimated one-third of known human estrogen-targeted genes associate only indirectly with ER, via intermediary transcription factors, and Sp1 is the predominant such mediator (reviewed in e.g., O'Lone et al. 2004). This induction pathway, referred to as transcription factor crosstalk, is one of four proposed processes underlying estrogen signaling (for review, see e.g. (Zhao et al., 2010)).
The identified key elements in the LDLR promoter for estrogen action are a sterol-responsive element (SRE), 5′-ATCACCCCAC-3′, and two Sp1 sites, 5′-CTCCTCC-3′ and 5′-CTCCTCCCC-3′ positioned upstream and downstream, respectively, of the SRE (Bruning et al., 2003; Li et al., 2001). Reminiscent of this arrangement of key elements in the LDLR's promoter, in addition to the presumptive (almost perfectly palindromic) ERE in the promoter of the ggLRP2 gene, 5′-TT GGTCA GCC TGAGC CT-3′, sthere are also 4 elements that display the Sp1-binding site signatures, 5′-TCCC/TT-3′ or 5′-TCCCCTCC-3′, of which 2 are upstream and 2 downtream of the ERE (Fig. 6B). These elements are binding sites for Sp1 and Sp3 proteins, which are capable of activating transcription (Li and Davie, 2010). A ggLRP2 promoter fragment containing all four Sp1-binding sites and the ERE was shown to induce transcription even without estrogen treatment, as determined by a luciferase activity assay (data not shown). It is known that the transcriptional induction of estrogen-responsive genes by estrogen can be achieved by orchestrating complex formation between estrogen receptor and Sp1 (Kim et al., 2003). Thus, in addition to direct estrogen induction via the classical ligand-induced ERE pathway, the indirect transcription factor crosstalk pathway engaging the Sp1 sites may well operate in mediating estrogen's action on the ggLRP2 gene. The addition of estrogen further increased the effect on transcriptional activation of the reporter gene (data not shown), indicating the functionality of the ggERE and its contribution to the complex gene regulation of the galline LRP2 gene. Future studies are aimed at delineating the type(s) of processes that mediate the estrogen-responsiveness of both human and galline LRP2 genes by detailed analysis of the promoter region.
Is there a specific function of chicken LRP2 that may require, or provide an advantage by, estrogen-regulation and/or higher renal expression in females? One possibility is that the higher levels of a receptor essential to retention of small proteins from the glomerular ultrafiltrate in mature females is required for coping with the grossly altered physiological state of hens induced by estrogen (Chan et al., 1976; Kirchgessner et al., 1987; Lazier et al., 1994; Luskey et al., 1974; O'Lone et al., 2004; Walzem et al., 1999). The most obvious changes occur in the levels of serum components, especially lipoproteins: VLDL levels rise 20-50-fold during sexual maturation (i.e., at onset of egg-laying), while the HDL fraction, predominant in roosters and immature females, becomes negligible. Retention of important proteins, such as apoA-I, the major apo of HDL, as well as that of low-molecular weight proteins including vitamin- and steroid-binding proteins by LRP2 (reviewed, e.g., in (Christensen et al., 1999; Willnow and Nykjaer, 2010)), may be particularly critical in the challenging physiological condition of the laying hen. In addition or alternatively, enhanced lipoprotein uptake into the kidney by LRP2 may be required to provide precursors for the enhanced synthesis of apo-VLDL-II-poor VLDL particles that supply extraoocytic target sites with energy (Walzem et al., 1999). In conclusion, control of hepatic biosynthetic genes and the renal LRP2 gene are tightly linked not only under chronically elevated estrogen levels, as in laying hens, but acute estrogen administration triggers the equivalent physiological changes and enhances renal expression of LRP2 in roosters.
Acknowledgments
We would like to thank Dr. Sato Kan, Tokyo University of Agriculture and Technology, Japan, for fruitful discussions about early sequence data, and Dr. Michael Jakl for help with the illustrations. This work was supported by the Austrian National Bank Jubileefund Project Nr. 10514 (M.H.), by Grants FWF P19680-B11 (M.H.), FWF P20218-B11 and the Austrian Ministry of Science and Research GEN-AU Grant GOLD-Genomics of Lipid-associated Disorders (W.J.S.), and by a DOC-fFORTE-fellowship of the Austrian Academy of Sciences (J.A.P.).
References
- Anzenberger U. Elucidation of megalin/LRP2-dependent endocytic transport processes in the larval zebrafish pronephros. J. Cell Sci. 2006;119:2127–2137. doi: 10.1242/jcs.02954. [DOI] [PubMed] [Google Scholar]
- Bergink E.W., Wallace R.A. Precursor–product relationship between amphibian vitellogenin and the yolk proteins, lipovitellin and phosvitin. J. Biol. Chem. 1974;249:2897–2903. [PubMed] [Google Scholar]
- Birn H. The kidney in vitamin B12 and folate homeostasis: characterization of receptors for tubular uptake of vitamins and carrier proteins. Am. J. Physiol. Renal Physiol. 2006;291:F22–F36. doi: 10.1152/ajprenal.00385.2005. [DOI] [PubMed] [Google Scholar]
- Blue M.L., Protter A.A., Williams D.L. Biosynthesis of apolipoprotein B in rooster kidney, intestine, and liver. J. Biol. Chem. 1980;255:10048–10051. [PubMed] [Google Scholar]
- Bruning J.C. Estrogen receptor-alpha and Sp1 interact in the induction of the low density lipoprotein-receptor. J. Steroid Biochem. Mol. Biol. 2003;86:113–121. doi: 10.1016/s0960-0760(03)00263-2. [DOI] [PubMed] [Google Scholar]
- Capony F., Williams D.L. Apolipoprotein B of avian very low density lipoprotein: characteristics of its regulation in nonstimulated and estrogen-stimulated rooster. Biochemistry. 1980;19:2219–2226. doi: 10.1021/bi00551a035. [DOI] [PubMed] [Google Scholar]
- Chan L., Jackson R.L., O'Malley B.W., Means A.R. Synthesis of very low density lipoproteins in the cockerel. Effects of estrogen. J. Clin. Invest. 1976;58:368–379. doi: 10.1172/JCI108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen E.I., Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 2002;3:256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- Christensen E.I., Gliemann J., Moestrup S.K. Renal tubule gp330 is a calcium binding receptor for endocytic uptake of protein. J. Histochem. Cytochem. 1992;40:1481–1490. doi: 10.1177/40.10.1382088. [DOI] [PubMed] [Google Scholar]
- Christensen E.I. Evidence for an essential role of megalin in transepithelial transport of retinol. J. Am. Soc. Nephrol. 1999;10:685–695. doi: 10.1681/ASN.V104685. [DOI] [PubMed] [Google Scholar]
- Christensen E.I. Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8472–8477. doi: 10.1073/pnas.1432873100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen E.I., Raciti D., Reggiani L., Verroust P.J., Brandli A.W. Gene expression analysis defines the proximal tubule as the compartment for endocytic receptor-mediated uptake in the Xenopus pronephric kidney. Pflugers Arch. 2008;456:1163–1176. doi: 10.1007/s00424-008-0488-3. [DOI] [PubMed] [Google Scholar]
- Codina-Salada J., Moore J.P., Chan L. Kinetics of primary and secondary stimulation of the mRNA for APOVLDL-II, a major yolk protein, in the cockerel liver by estrogen. Endocrinology. 1983;113:1158–1160. doi: 10.1210/endo-113-3-1158. [DOI] [PubMed] [Google Scholar]
- Dolmer K., Gettins P.G. Three complement-like repeats compose the complete alpha2-macroglobulin binding site in the second ligand binding cluster of the low density lipoprotein receptor-related protein. J. Biol. Chem. 2006;281:34189–34196. doi: 10.1074/jbc.M604389200. [DOI] [PubMed] [Google Scholar]
- Gliemann J. Receptors of the low density lipoprotein (LDL) receptor family in man. Multiple functions of the large family members via interaction with complex ligands. Biol. Chem. 1998;379:951–964. [PubMed] [Google Scholar]
- Graham F.L., Smiley J., Russell W.C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hammes A. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122:751–762. doi: 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Harnish D.C., Evans M.J., Scicchitano M.S., Bhat R.A., Karathanasis S.K. Estrogen regulation of the apolipoprotein AI gene promoter through transcription cofactor sharing. J. Biol. Chem. 1998;273:9270–9278. doi: 10.1074/jbc.273.15.9270. [DOI] [PubMed] [Google Scholar]
- Hermann M., Seif F., Schneider W.J., Ivessa N.E. Estrogen dependence of synthesis and secretion of apolipoprotein B-containing lipoproteins in the chicken hepatoma cell line, LMH-2A. J. Lipid Res. 1997;38:1308–1317. [PubMed] [Google Scholar]
- Herz J., Strickland D.K. LRP: a multifunctional scavenger and signaling receptor. J. Clin. Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalm G. Cloning and sequencing of human gp330, a Ca(2 +)-binding receptor with potential intracellular signaling properties. Eur. J. Biochem. 1996;239:132–137. doi: 10.1111/j.1432-1033.1996.0132u.x. [DOI] [PubMed] [Google Scholar]
- Hofmeister M.V. 17beta-Estradiol induces nongenomic effects in renal intercalated cells through G protein-coupled estrogen receptor 1. Am. J. Physiol. Renal Physiol. 2012;302:F358–F368. doi: 10.1152/ajprenal.00343.2011. [DOI] [PubMed] [Google Scholar]
- Hummel S., Lynn E.G., Osanger A., Hirayama S., Nimpf J., Schneider W.J. Molecular characterization of the first avian LDL receptor: role in sterol metabolism of ovarian follicular cells. J. Lipid Res. 2003;44:1633–1642. doi: 10.1194/jlr.M300014-JLR200. [DOI] [PubMed] [Google Scholar]
- Jost J.P., Moncharmont B., Jiricny J., Saluz H., Hertner T. In vitro secondary activation (memory effect) of avian vitellogenin II gene in isolated liver nuclei. Proc. Natl. Acad. Sci. U. S. A. 1986;83:43–47. doi: 10.1073/pnas.83.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M.G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc. Natl. Acad. Sci. U. S. A. 1982;79:5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M.G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J. Exp. Med. 1983;157:667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Thu N., Saville B., Safe S. Domains of estrogen receptor alpha (ERalpha) required for ERalpha/Sp1-mediated activation of GC-rich promoters by estrogens and antiestrogens in breast cancer cells. Mol. Endocrinol. 2003;17:804–817. doi: 10.1210/me.2002-0406. [DOI] [PubMed] [Google Scholar]
- Kirchgessner T.G. Regulation of chicken apolipoprotein B: cloning, tissue distribution, and estrogen induction of mRNA. Gene. 1987;59:241–251. doi: 10.1016/0378-1119(87)90332-5. [DOI] [PubMed] [Google Scholar]
- Klinge C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas M.Z. Identification of glycoprotein 330 as an endocytic receptor for apolipoprotein J/clusterin. J. Biol. Chem. 1995;270:13070–13075. doi: 10.1074/jbc.270.22.13070. [DOI] [PubMed] [Google Scholar]
- Kummer S. Estrogen receptor alpha expression in podocytes mediates protection against apoptosis in-vitro and in-vivo. PLoS One. 2011;6:e27457. doi: 10.1371/journal.pone.0027457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazier C.B., Wiktorowicz M., DiMattia G.E., Gordon D.A., Binder R., Williams D.L. Apolipoprotein (apo) B and apoII gene expression are both estrogen-responsive in chick embryo liver but only apoII is estrogen-responsive in kidney. Mol. Cell. Endocrinol. 1994;106:187–194. doi: 10.1016/0303-7207(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Leheste J.R. Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J. 2003;17:247–249. doi: 10.1096/fj.02-0578fje. [DOI] [PubMed] [Google Scholar]
- Li L., Davie J.R. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann. Anat. 2010;192:275–283. doi: 10.1016/j.aanat.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Li C., Briggs M.R., Ahlborn T.E., Kraemer F.B., Liu J. Requirement of Sp1 and estrogen receptor alpha interaction in 17beta-estradiol-mediated transcriptional activation of the low density lipoprotein receptor gene expression. Endocrinology. 2001;142:1546–1553. doi: 10.1210/endo.142.4.8096. [DOI] [PubMed] [Google Scholar]
- Liu W. Regulation of gp330/megalin expression by vitamins A and D. Eur. J. Clin. Invest. 1998;28:100–107. doi: 10.1046/j.1365-2362.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- Luskey K.L., Brown M.S., Goldstein J.L. Stimulation of the synthesis of very low density lipoproteins in rooster liver by estradiol. J. Biol. Chem. 1974;249:5939–5947. [PubMed] [Google Scholar]
- Marino M., Andrews D., Brown D., McCluskey R.T. Transcytosis of retinol-binding protein across renal proximal tubule cells after megalin (gp 330)-mediated endocytosis. J. Am. Soc. Nephrol. 2001;12:637–648. doi: 10.1681/ASN.V124637. [DOI] [PubMed] [Google Scholar]
- Marzolo M.P., Farfan P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol. Res. 2011;44:89–105. doi: 10.4067/S0716-97602011000100012. [DOI] [PubMed] [Google Scholar]
- Mii A. Genetic association of low-density lipoprotein receptor-related protein 2 (LRP2) with plasma lipid levels. J. Atheroscler. Thromb. 2007;14:310–316. doi: 10.5551/jat.e494. [DOI] [PubMed] [Google Scholar]
- Moestrup S.K., Verroust P.J. Megalin- and cubilin-mediated endocytosis of protein-bound vitamins, lipids, and hormones in polarized epithelia. Annu. Rev. Nutr. 2001;21:407–428. doi: 10.1146/annurev.nutr.21.1.407. [DOI] [PubMed] [Google Scholar]
- Muller D., Nykjaer A., Willnow T.E. From holoprosencephaly to osteopathology: role of multifunctional endocytic receptors in absorptive epithelia. Ann. Med. 2003;35:290–299. doi: 10.1080/07853890310006488. [DOI] [PubMed] [Google Scholar]
- Norden A.G. Urinary megalin deficiency implicates abnormal tubular endocytic function in Fanconi syndrome. J. Am. Soc. Nephrol. 2002;13:125–133. doi: 10.1681/ASN.V131125. [DOI] [PubMed] [Google Scholar]
- Noteborn M.H., Bakker O., de Jonge M.A., Gruber M., Ab G. Differential estrogen responsiveness of the vitellogenin and apo very low density lipoprotein II genes in the rooster liver. J. Steroid Biochem. 1986;24:281–285. doi: 10.1016/0022-4731(86)90065-8. [DOI] [PubMed] [Google Scholar]
- Nykjaer A., Willnow T.E. The low-density lipoprotein receptor gene family: a cellular Swiss army knife? Trends Cell Biol. 2002;12:273–280. doi: 10.1016/s0962-8924(02)02282-1. [DOI] [PubMed] [Google Scholar]
- Nykjaer A. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3) Proc. Natl. Acad. Sci. U. S. A. 2001;98:13895–13900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogburn C.E. Cultured renal epithelial cells from birds and mice: enhanced resistance of avian cells to oxidative stress and DNA damage. J. Gerontol. A Biol. Sci. Med. Sci. 1998;53:B287–B292. doi: 10.1093/gerona/53a.4.b287. [DOI] [PubMed] [Google Scholar]
- O'Lone R., Frith M.C., Karlsson E.K., Hansen U. Genomic targets of nuclear estrogen receptors. Mol. Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- Orlando R.A. Identification of the second cluster of ligand-binding repeats in megalin as a site for receptor-ligand interactions. Proc. Natl. Acad. Sci. U. S. A. 1997;94:2368–2373. doi: 10.1073/pnas.94.6.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen G.A., Chakraborty S., Steinhauser A.L., Traub L.M., Madsen M. AMN directs endocytosis of the intrinsic factor-vitamin B(12) receptor cubam by engaging ARH or Dab2. Traffic. 2010;11:706–720. doi: 10.1111/j.1600-0854.2010.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwon N., Gunther W., Schwake M., Bosl M.R., Jentsch T.J. ClC-5 Cl−-channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature. 2000;408:369–373. doi: 10.1038/35042597. [DOI] [PubMed] [Google Scholar]
- Saito A., Pietromonaco S., Loo A.K., Farquhar M.G. Complete cloning and sequencing of rat gp330/“megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc. Natl. Acad. Sci. U. S. A. 1994;91:9725–9729. doi: 10.1073/pnas.91.21.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W.J. Low density lipoprotein receptor relatives in chicken ovarian follicle and oocyte development. Cytogenet. Genome Res. 2007;117:248–255. doi: 10.1159/000103186. [DOI] [PubMed] [Google Scholar]
- Schneider W.J. Receptor-mediated mechanisms in ovarian follicle and oocyte development. Gen. Comp. Endocrinol. 2009;163:18–23. doi: 10.1016/j.ygcen.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Staels B., Auwerx J., Chan L., van Tol A., Rosseneu M., Verhoeven G. Influence of development, estrogens, and food intake on apolipoprotein A-I, A-II, and E mRNA in rat liver and intestine. J. Lipid Res. 1989;30:1137–1145. [PubMed] [Google Scholar]
- Stefansson S., Chappell D.A., Argraves K.M., Strickland D.K., Argraves W.S. Glycoprotein 330/low density lipoprotein receptor-related protein-2 mediates endocytosis of low density lipoproteins via interaction with apolipoprotein B100. J. Biol. Chem. 1995;270:19417–19421. doi: 10.1074/jbc.270.33.19417. [DOI] [PubMed] [Google Scholar]
- Stifani S., George R., Schneider W.J. Solubilization and characterization of the chicken oocyte vitellogenin receptor. Biochem. J. 1988;250:467–475. doi: 10.1042/bj2500467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarugi P., Ballarini G., Pinotti B., Franchini A., Ottaviani E., Calandra S. Secretion of apoB- and apoA-I-containing lipoproteins by chick kidney. J. Lipid Res. 1998;39:731–743. [PubMed] [Google Scholar]
- Treilleux, Peloux N., Brown M., Sergeant A. Human estrogen receptor (ER) gene promoter-P1: estradiol-independent activity and estradiol inducibility in ER + and ER − cells. Mol. Endocrinol. 1997;11:1319–1331. doi: 10.1210/mend.11.9.9973. [DOI] [PubMed] [Google Scholar]
- Vegt E. Renal uptake of different radiolabelled peptides is mediated by megalin: SPECT and biodistribution studies in megalin-deficient mice. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:623–632. doi: 10.1007/s00259-010-1685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzem R.L., Hansen R.J., Williams D.L., Hamilton R.L. Estrogen induction of VLDLy assembly in egg-laying hens. J. Nutr. 1999;129:467S–472S. doi: 10.1093/jn/129.2.467S. [DOI] [PubMed] [Google Scholar]
- Wang S.Y., Williams D.L. Differential responsiveness of avian vitellogenin I and vitellogenin II during primary and secondary stimulation with estrogen. Biochem. Biophys. Res. Commun. 1983;112:1049–1055. doi: 10.1016/0006-291x(83)91724-2. [DOI] [PubMed] [Google Scholar]
- Willnow T.E., Nykjaer A. Cellular uptake of steroid carrier proteins—mechanisms and implication. Mol. Cell. Endocrinol. 2010;316:93–102. doi: 10.1016/j.mce.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Willnow T.E. Defective forebrain development in mice lacking gp330/megalin. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8460–8464. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R.F., Gordon J.I., Deeley R.G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc. Natl. Acad. Sci. U. S. A. 1980;77:4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J., Greenwald I. A gene for a low density lipoprotein receptor-related protein in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 1993;90:4572–4576. doi: 10.1073/pnas.90.10.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J., Tuck S., Greenwald I., Han M. A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development. 1999;126:597–606. doi: 10.1242/dev.126.3.597. [DOI] [PubMed] [Google Scholar]
- Yuseff M.I., Farfan P., Bu G., Marzolo M.P. A cytoplasmic PPPSP motif determines megalin's phosphorylation and regulates receptor's recycling and surface expression. Traffic. 2007;8:1215–1230. doi: 10.1111/j.1600-0854.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Zhao C., Dahlman-Wright K., Gustafsson J.A. Estrogen signaling via estrogen receptor {beta} J. Biol. Chem. 2010;285:39575–39579. doi: 10.1074/jbc.R110.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpha 2MR, and the receptor-associated protein (RAP) J. Histochem. Cytochem. 1994;42:531–542. doi: 10.1177/42.4.7510321. [DOI] [PubMed] [Google Scholar]