Figure 6.
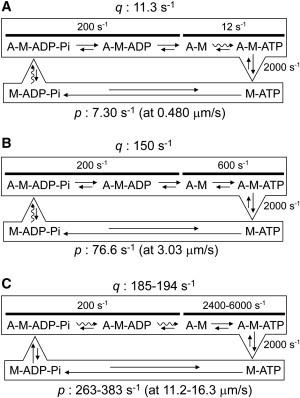
Summary of the actomyosin ATPase cycle and kinetic parameters. The wavy arrows in the ATPase cycle represent the probable rate-limiting step. (A and B) Kinetic parameters at the low HMM density described in this study. The dissociation rate constants q at (A) 10 μM and (B) 500 μM ATP were calculated from Eq. 1. The values of the association rate constants p were obtained in this study. (C) Kinetic parameters at high HMM density estimated using the values for ATP concentration and sliding velocity reported in Harada et al. ((3); 2 mM ATP, 11.2 μm/s at 30°C, in vitro motility) and Pate et al. (31); 5 mM ATP, 16.3 μm/s at 30°C, muscle fiber). The association rate constant p was calculated using the slope of Fig. 5B and the sliding velocity reported in (3,31).
