Abstract
Purpose
Rhegmatogenous retinal detachment (RRD) affects the function of the retina before and after surgical repair. We investigated ultra-wide-field autofluorescence (UAF) abnormalities in patients with acute RRD to improve our understanding of the functional changes in the retina before and after surgery.
Methods
In this retrospective study, we present the UAF imaging findings of 16 patients with acute, non-traumatic RRD. Imaging was obtained with the Optos 200 Tx (Optos) in 14 eyes preoperatively and in 12 eyes postoperatively. Twelve eyes had RRDs that involved the macula (group A), whereas four eyes had macula-sparing RRDs (group B).
Results
All patients (100%) with bullous retinal detachments demonstrated hypofluorescence over the area of retinal detachment. A hyperfluorescent leading edge (HLE) to the retinal detachment was observed preoperatively in 100% of eyes in group A and 75% of eyes in group B. Preoperative UAF through the fovea of group A eyes was normal (30%), hypofluorescent (50%) or hyperfluorescent (20%). In all patients with a HLE preoperatively, the HLE resolved by the 1-month postoperative visit. A residual line of demarcation remained in 8 of the 12 eyes (67%). In group A eyes, postoperative granular autofluorescent changes were present in four of the nine (44%) eyes, and were associated with worse preoperative (P=0.04) and postoperative (P=0.09) visual acuity.
Conclusion
UAF imaging reveals abnormalities in RRDs that allow excellent demarcation of the extent of the retinal detachment and assist in preoperative characterization of the detachment and postoperative counselling.
Keywords: autofluorescence, imaging, retinal detachment, ultra-wide-field
Introduction
Rhegmatogenous retinal detachment (RRD) is an important cause of visual impairment and blindness. Despite the fact that anatomical success rates have improved dramatically over the past century, with ∼90% or more of all RRDs successfully repaired with one or more operations,1, 2, 3 postoperative visual acuity (VA) outcomes continue to be unpredictable in many patients.
Several causes of decreased VA after successful repair of retinal detachment have been identified, and these include persistent foveal detachment,4 epiretinal membrane, cystoid macular oedema,5, 6 and retinal folds.7 With improved visualization techniques, such as optical coherence tomography (OCT), structural abnormalities of the retina can be readily identified postoperatively; however, the functional status of the retina remains difficult to assess.
Clinical autofluorescence (AF) is an imaging modality that is able to evaluate the functional status of the retina, in addition to the anatomy. AF imaging measures the amount of fluorescent light emitted from a structure when stimulated by light of a shorter wavelength. In the human retina, the majority of the AF signal is derived from lipofuscin in the retinal pigment epithelium (RPE).8, 9 The quantity of lipofuscin in the RPE reflects a balance between accumulation and clearance, and is dependent upon photoreceptor outer segment turnover and retinoid cycling.9 Thus, AF is a valuable modality for assessing the function of RPE cells and the viability of retina.
In the current study, we used ultra-wide-field AF (UAF) to assess patients with RRD before and after surgical repair in order to determine the AF abnormalities of these patients. Currently, fundus AF imaging can be obtained using a confocal scanning laser ophthalmoscope, such as provided in the Heidelberg Spectralis (Heidelberg Engineering Inc., Heidelberg, Germany). This produces a 30° or 50° view of the retina and uses a blue excitation light (488 nm) to obtain AF images. Ultra-wide-field imaging (Optos plc, Dunfermline, UK), by contrast, offers a 200° view of the retina on a single frame, which allows simultaneous visualization of the AF levels in the retinal fundus and periphery. The AF signal obtained with this technology results from a green excitation light (532 nm). We believe ultra-wide-field imaging is more suitable for imaging retinal detachments because RRDs typically result from a peripheral retinal break and extend posteriorly.
Materials and methods
This retrospective, observational study was performed after obtaining Institutional Review Board approval at the Weill Cornell Medical College in New York, NY, USA. All the medical records of the patients diagnosed with acute (duration of symptoms ≤ 30 days), primary, non-traumatic RRD at the Weill Cornell Eye Associates between August 2010 and September 2011 were reviewed. Only those who had UAF (Optos 200 Tx, Optos plc) imaging were included.
The Optos 200 Tx uses red (633 nm) and green (532 nm) laser wavelengths to produce a digital, high-resolution image that is displayed on a monitor screen. For the laser input path, in AF mode, the red laser is blocked by a shutter and the eye is only illuminated by the green laser. In this mode, polarization discrimination optics are moved out of the return path (there is no reflection from the cornea) to optimize the detector collection of the AF component. AF images were obtained using a 532-nm wavelength green light for excitation, whereas detection of the emitted signal occurs using a raster scan and detector for light in the wavelengths from 570 to 780 nm (yellow-orange-red range) in a standardized manner. Standardization consisted of full pharmacologic dilation before obtaining the images. The photographer obtained several images to obtain optimal focusing of the macula during the AF imaging.
All patients underwent pre- and postoperative VA testing, slit lamp biomicroscopy, and dilated fundus examination. All patients with macula-involving RRD (group A) also underwent spectral domain OCT (SD-OCT) using the Spectralis HRA+OCT (Heidelberg Engineering Inc.). Other data collected included demographics, duration of symptoms, presence of foveal involvement, time to surgery, type of surgical repair, and complications. Patients with other retinal diseases that can potentially change the AF findings, such as diabetic retinopathy, retinal vein occlusions, and chorioretinal dystrophies, were excluded. The typical postoperative follow-up visits for the patients included a visit 1 day, 1 week, and 1 month after the date of surgery. AF imaging was typically performed preoperatively and at the 1-month postoperative visit. AF characteristics were analysed qualitatively. The AF patterns were compared with the normal, fellow eye. Interpretation of AF imaging was performed in all patients with preoperative imaging and/or 1-month postoperative imaging. Only the preoperative and 1-month postoperative images were evaluated. Best corrected Snellen VA was converted to logMAR (logarithm of the minimum angle of resolution) for statistical analysis. The following conversion to logMAR was used for vision worse than 20/400: counting fingers=1.6, hand motion=2.0, light perception 2.5, and no light perception=3.0.
Statistical analysis
Statistical testing to compare the pre- and postoperative VAs of patients with postoperative granular AF changes in this study was performed using an unpaired t-test to compare means and yield P-values. P-values <0.05 were considered statistically significant.
Results
Sixteen patients (16 eyes) with acute (duration of symptoms ≤30 days) onset of RRD were included in the final analysis. Table 1 summarizes the data from the patients included in this study. The images were obtained at a mean of 1.57 days preoperatively (range 0–7 days). The patients presented with symptoms for an average of 10.4 days (range 1–30 days). Twelve of these eyes were diagnosed with macula-involving detachments (group A), whereas four of the eyes had macula-sparing detachments (group B).
Table 1. Demographics.
| N or mean | Range | |
|---|---|---|
| Total eyes | 16 | |
| Group A—macula involving | 12 | |
| Group B—macula sparing | 4 | |
| Group A | 12 | |
| Age, years | 62.7 | 47–98 |
| Male | 6 | |
| Right eyes | 5 | |
| Duration of symptoms, days | 11 | 3–30 |
| Fovea attached on OCT | 2 | |
| Preoperative logMAR | 0.9 | |
| One-month postoperative logMAR | 0.52 | |
| Surgical treatment | ||
| PPV | 12 | |
| Group B | 4 | |
| Age, years | 43.5 | 25–62 |
| Male | 3 | |
| Right eyes | 3 | |
| Duration of symptoms, days | 9 | 1–14 |
| Preoperative logMAR | 0.02 | |
| One-month postoperative logMAR | 0.16 | |
| Surgical treatment | ||
| PPV | 2 | |
| SB | 2 | |
Abbreviations: logMAR, logarithm of minimum angle of resolution; PPV, pars plana vitrectomy; SB, scleral buckle.
Preoperative AF
Preoperative AF imaging was obtained in 14 eyes; 10 in group A and 4 in group B. The findings from these images are displayed in Table 2. All patients with a clinical bullous retinal detachment on examination demonstrated hypofluorescence over the retinal detachment on AF imaging (9/9 in group A and 3/3 in group B; Figures 1 and 2). A hyperfluorescent leading edge (HLE) to the retinal detachment was present in all (100%) of the eyes in group A (Figures 1 and 2) and 75% of the eyes in group B. In each case, the HLE extended peripherally to the extent of the clinical retinal detachment. OCT of each of the eyes in group A demonstrated that the HLE corresponded to areas of neurosensory detachment and shallow subretinal fluid, which was continuous with the retinal detachment (Figures 1 and 2). In two eyes in group A, the HLE passed directly through the fovea.
Table 2. Pre- and postoperative AF characteristics.
| N | Percentages | |
|---|---|---|
| Total eyes with preoperative UAF imaging | 14 | |
| Group A | 10 | |
| Bullous retinal detachment on clinical exam | 9 | |
| Hypofluorescence over bullous RD | 9 | 100% |
| HLE present | 10 | 100% |
| HLE through fovea | 2 | 20% |
| AF in fovea | 10 | Mean VA |
| Normal | 3 | 0.40 |
| Hyper | 2 | 0.45 |
| Hypo | 5 | 1.42 |
| Group B | 4 | |
| Bullous retinal detachment on clinical exam | 3 | |
| Hypofluorescence over bullous RD | 3 | 100% |
| HLE present | 3 | 75% |
| HLE through fovea | 0 | 0% |
| AF in fovea | 4 | |
| Normal | 4 | 100% |
| Total eyes with postoperative UAF imaging | 12 | |
| Group A | 9 | |
| Resolution of HLE with preoperative HLE (n=7) | 7 | 100% |
| Line of demarcation (n=9) | 6 | 67% |
| AF of fovea | 9 | |
| Normal | 7 | 78% |
| Hyperfluorescent spot | 2 | 22% |
| Granular AF in macula | 4 | 44% |
| Group B | 3 | |
| Resolution of HLE with preoperative HLE (n=2) | 2 | 100% |
| Line of demarcation (n=3) | 2 | 67% |
| AF of fovea | 3 | |
| Normal | 3 | 100% |
| Granular AF in macula | 0 | 0% |
Abbreviations: AF, autofluroscence; HLE, hyperfluorescent leading edge; VA, visual acuity.
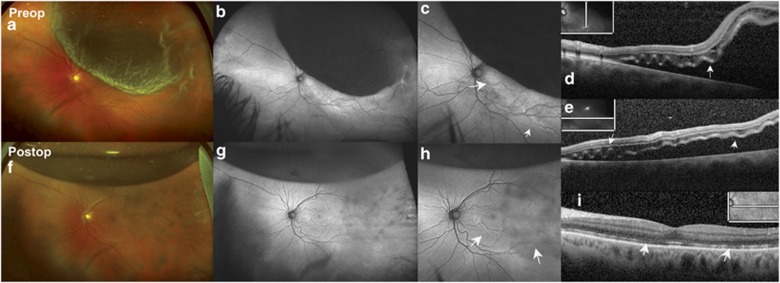
Figure 1.
A 64-year-old male patient with 3 days of symptoms presented with a fovea-involving RRD. (a) Preoperative ultra-wide-field colour fundus photograph of the left eye shows superotemporal macula-involving RRD with outer retinal corrugations and retinal breaks visualized temporally. (b) UAF of the left eye shows hypofluorescence over the bullous RD and a HLE. (c) Magnified AF image of the left macula shows the HLE in more detail (arrows). (d) Preoperative OCT shows CME in the outer nuclear layers and submacular fluid and undulations in outer retina (arrows). (e) Preoperative OCT of a horizontal scan showing similar changes as in panel (d). (f) Postoperative ultra-wide-field colour fundus photograph 1 month after the repair shows attached retina with residual C3F8 gas bubble superiorly and chorioretinal scarring temporally from intraoperative endolaser. (g) Postoperative AF image shows persistent line of demarcation in the macula and granular areas of AF in the macular areas in sites of prior detachment, magnified in panel (h) (arrows). (i) Postoperative OCT shows resolution of CME and outer retina corrugation but persistent disruption of the outer retina in some areas (arrows). The vision improved from finger counting preoperatively to 20/40 postoperatively.
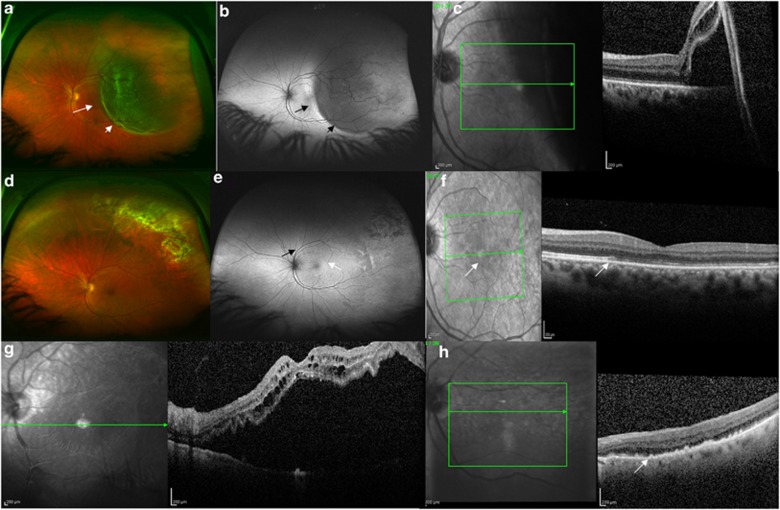
Figure 2.
(a) Preoperative wide-field colour image of a 50-year-old female patient with a RRD involving the fovea. The short arrow identifies the area of bullous retinal detachment readily identified with ophthalmoscopy. The long arrow identifies an area of shallow neurosensory detachment extending through the fovea. (b) Preoperative wide field AF image of the same patient demonstrating the area of bullous detachment as hypofluorescence (short arrow) and the HLE with the long black arrow, indicating the area of shallow neurosensory detachment. (c) Preoperative OCT image of the patient from panels (a) and (b). The boundary between detached and attached retina is readily identified. (d) One-month postoperative colour image demonstrating reattachment of the retina with laser chorioretinal scars in the superotemporal quadrant. (e) One-month peripheral AF image demonstrating the residual demarcation line (black arrow) and granular AF changes (white arrow) within the area of previously detached retina. (f) Postoperative OCT image of the patient. A white arrow on the infrared image highlights the boundary between attached and detached retina, preoperatively. The OCT shows disruption of the IS/OS junction in the region of previously detached retina (which showed granular changes with AF) and a white arrow shows the boundary. (g) Preoperative OCT image of a different patient (78-year-old male) with a macula-affecting retinal detachment. (h) Postoperative OCT image of the patient from panel (g), who demonstrated postoperative granular AF changes, demonstrating significant outer retinal disturbance. The outer retinal changes of the IS/OS junction appear similar to the subretinal or outer retinal deposits (white arrow).
In group A, the AF through the fovea was classified into one of the following three categories with respect to the patient's fellow eye: normal, hyperfluorescent, or hypofluorescent. Three eyes had normal AF, two were hyperfluorescent, and five were hypofluorescent. The mean preoperative VA in logMAR was 0.40, 0.45, and 1.42 for normal AF, hyperfluorescence, and hypofluorescence in the fovea, respectively.
Surgery
All 12 eyes in group A were treated with pars plana vitrectomy. Two eyes in group B were treated with a scleral buckling procedure and two eyes were treated with pars plana vitrectomy. All pars plana vitrectomies concluded with injection of an intraocular gas tamponade with 16% C3F8 gas. The scleral buckling procedures did not include the injection of intraocular gas or drainage of subretinal fluid. No procedures involved a combination with cataract extraction. In each of the patients included in the study, the surgical procedure was the primary surgical procedure for repair of the RRD (Table 1).
Postoperative AF
Postoperative AF images were obtained in 12 eyes; 9 eyes in group A and 3 in group B. The findings from these images are displayed in Table 2. These images were taken at a mean of 42 days postoperatively (range 21–90 days).
In all, 7 of the 10 eyes in group A with preoperative AF imaging had postoperative imaging. Three of the four eyes in group B had postoperative imaging. In group A, 1-month postoperative AF revealed the resolution of the hyperfluorescent edge of the retinal detachment in all eyes that had undergone pre- and postoperative imaging (7/7, 100%). In group B, the HLE had resolved at the 1-month postoperative visit in two of the two eyes (100%) that had a HLE preoperatively and had obtained pre- and postoperative imaging. Both of these eyes had been treated with pars plana vitrectomy. An additional two eyes with macula-affecting RRDs (group A) had postoperative AF imaging without preoperative imaging.
A residual line of demarcation was present with AF imaging at the 1-month postoperative visit in six eyes (6/9, 67%) in group A (Figures 1 and 2). These eyes were imaged an average of 46 days after the surgery. In group B, two eyes (2/3, 67%) demonstrated a line of demarcation.
Postoperatively, the AF images of the fovea appeared normal in 78% (7/9) in group A and 100% (3/3) in group B. One eye in group A had a hyperfluorescent spot in the fovea associated with subretinal fluid on the OCT and one eye demonstrated a hyperfluorescent spot associated with cystoid macular oedema on the OCT. The maculas of four eyes (4/9, 44%) in group A demonstrated AF with a granular pattern postoperatively in the area of previously detached retina (Figures 1 and 2). In each of these eyes, the fovea and macula were detached preoperatively. Group A eyes with granular AF changes on postoperative AF had worse preoperative VA compared with group A eyes with postoperative AF without granular changes (mean logMAR 1.28 vs 0.51, P=0.04). In addition, group A eyes with granular AF changes on postoperative AF had worse postoperative VA compared with group A eyes with postoperative AF without granular changes (mean logMAR 0.71 vs 0.31, P=0.09).
Discussion
Retinal AF is attributable to the fluorophores in the retina, the most predominant of which is lipofuscin.8 Increased levels of AF indicate increased presence of oxidative stress and increased metabolic activity as the RPE enters a preapoptotic state.9 Therefore, AF imaging serves as an indicator of retinal function.
Many reports investigating AF abnormalities in RRD have focused on the central macular areas.10, 11 The current report used an ultra-wide-field camera that allows a 200° view of the retina with a single frame. Peripheral views of the retina are valuable in imaging RRDs because this condition is a peripheral disease (ie, results from a retinal break in the periphery that may progress posteriorly). This report investigated the pre- and postoperative AF signal in eyes with RRDs.
The preoperative AF imaging of patients with RRD revealed several consistent findings. These included hypofluorescence in areas of bullous retinal detachment and a HLE in the majority of RRDs.
Hypofluorescence in areas of clinical, bullous retinal detachment (Figures 1 and 2) was found in every patient included in this study with a bullous RRD (12/12, 100%). This phenomenon is likely due to a blocking effect by the oedematous retina and subretinal fluid overlying the RPE. In two cases in this report, there was an absence of hypofluorescence overlying the retinal detachment (Figure 3). In both the cases, the retinal detachment appeared very shallow on clinical examination.
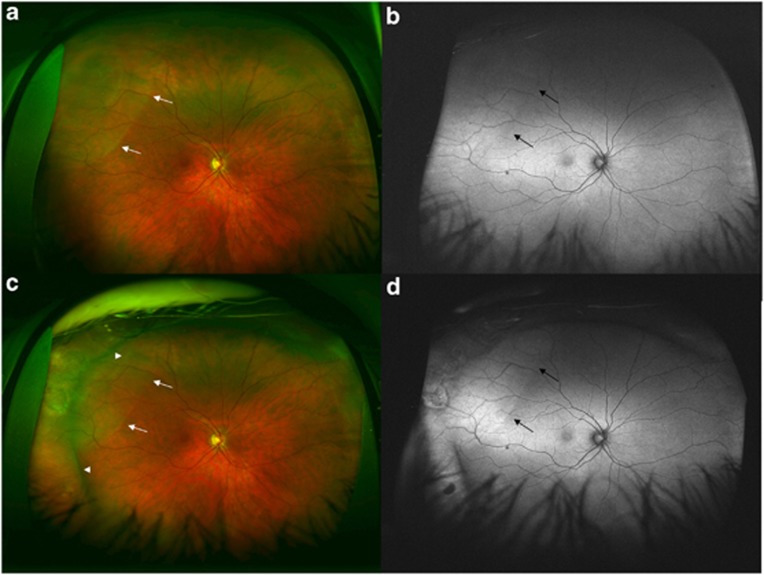
Figure 3.
A 46-year-old male patient presented with symptoms of flashes and floaters for 2 weeks and with a macula-sparing RRD of right eye. His presenting vision was 20/20. (a) The preoperative ultra-wide-field colour photo revealed a shallow RRD superotemporal to the macula. White arrows detail the extent of the RRD. (b) The preoperative UAF shows a transparent RRD and a lack of hypofluorescence over the RRD, with subtle hyperfluorescence over the RRD. Black arrows detail the extent of the RRD. (c) Patient was treated with a scleral buckle. One-month postoperative ultra-wide-field colour image demonstrates persistence of subretinal fluid superotemporally. The long arrows illustrate the extent of the subretinal fluid. The arrowheads illustrate the elevation from the scleral buckle. (d) Ultra-wide-field postoperative AF image shows more hyperfluorescent zone of subretinal fluid and clear demarcation from attached retina. Black arrows indicate the extent of the persistent subretinal fluid. The postoperative VA was 20/25.
A HLE (Figures 1 and 2), which was continuous with the retinal detachment, but extended beyond the extent of the clinical retinal detachment, was present in 93% (13/14) of the cases in our series. In each case, the HLE corresponded with the presence of shallow subretinal fluid upon OCT imaging. The HLE likely represents the collection of fluorophores from photoreceptor outer segments, which have been shed and are collecting in the subretinal space. This would be the most likely explanation given the OCT findings; however, it may also represent RPE cells with increased metabolic activity due to the stress at the border of attached and detached retina.
Twelve eyes in this report were imaged postoperatively with AF imaging. Several characteristics were present in these patients, including a residual line of AF demarcation, hyperfluorescent changes adjacent to retinal vessels, hyperfluorescent macular changes due to pathology, granular AF changes in the macula, and residual hyperfluorescence after scleral buckling procedure.
A residual line of demarcation was frequently located postoperatively in the location of the prior HLE (ie, shallow neurosensory detachment; Figures 1 and 2). This line of demarcation, however, was only seen in eyes that had demonstrated a HLE preoperatively. It is possible that both the HLE and residual line of demarcation are more common in eyes with RRD that is acute. The duration of this line is unknown; however, one eye in the study demonstrated the line of demarcation at 1 month of follow-up and it had disappeared 6½ months after the surgery. In another eye, which was imaged 4 months postoperatively, the line of demarcation persisted. The location of this line was critical in determining the preoperative AF characteristics of the fovea. If the line went directly through the fovea, the fovea appeared hyperfluorescent, whereas if the detachment involved the macula, but had not reached the fovea yet, the AF of the fovea continued to appear normal.
Two eyes in the study demonstrated hyperfluorescent lines parallel to the retinal vessels postoperatively (Figure 1). This is similar to the phenomenon described in the report by Shiragami et al.11 As suggested in the report, these hyperfluorescent lines may develop because the retina is reattached in a slightly different position postoperatively than where it had been before the retinal detachment. These lines remained in one eye for 4 months postoperatively, but had disappeared by 11 months.
Two patients in this report exhibited hyperfluorescent areas in the macula postoperatively. One patient was found to have subretinal fluid and the other demonstrated cystoid macular oedema when imaged with the OCT. Cystoid macular oedema has been identified on AF imaging in the location of thinning, depigmentation, or incipient atrophy of the foveal RPE.9 The intensity of AF changes depends upon the status of the overlying retina because AF generated by the RPE cells travels through the sensory retina.12, 13 Hyperfluorescence, therefore, may also result from increased transmission due to retinal atrophy or subretinal fluid leading to separation of the outer segments of the photoreceptors from the underlying RPE, causing improper outer segment turnover, intraretinal fluid, and displacement of luteal pigment.12
Diffuse granular hyperfluorescence was present in four eyes postoperatively that had presented with macula-affecting retinal detachments (group A; Figures 1 and 2). These changes appeared to persist the length of follow-up and seemed to be most prominent in the area of retina that had been detached preoperatively. The prior edge of hyperfluorescence seemed to represent the extent to which the granular changes extended into uninvolved macula. OCT imaging of eyes with granular AF changes postoperatively revealed significant disruption of the outer retinal anatomy. Specifically, the IS/OS junction was typically disrupted (Figure 2). In some cases, the IS/OS junction was so discontinuous that it appeared as if subretinal deposits were present on the OCT image. (Figure 2h). There was a correlation between postoperative AF abnormalities and OCT findings. Within group A, the patients with granular macular changes had a statistically significant worse mean VA preoperatively (logMAR 1.28 vs 0.51, P=0.04) and a trend for worse VA postoperatively (logMAR 0.71 vs 0.31, P=0.09) compared with the eyes in group A without postoperative granular AF macular changes.
Postoperative AF imaging was obtained in one eye that had repair of a macula-sparing RRD with a scleral buckle (Figure 3). At the 1-month postoperative visit, this eye demonstrated residual subretinal fluid clinically and hyperfluorescence in the same location as preoperative imaging (Figure 3). AF imaging was particularly useful postoperatively in detecting the presence of subretinal fluid and delineating its borders. This imaging modality may be useful in monitoring the resorption of subretinal fluid after scleral buckling procedures.
The limitations of this study include the retrospective design of the study, inherent limitations of the Optos 200 Tx imaging technology, small sample size, and lack of comparison between UAF images (obtained using the Optos 200 Tx) and conventional AF images (using the Heidelberg Spectralis). The limitations of the Optos imaging platform include the fact that the Optos may fail to adequately image pathology in the superior and inferior peripheral retina,14 and the dependency of AF imaging to rely on precise focusing by the photographer. The small sample size limits rigorous statistical analysis; however, several consistent findings of eyes with RRD using AF imaging are outlined in this report.
This is the first paper to evaluate RRD in eyes using UAF imaging. These images provide a surgeon with a greater appreciation of the extent of the retinal detachment preoperatively. In conjunction with the use of OCT, AF images may also aid in classification of RRD preoperatively into macula-involving vs macula-sparing detachments and fovea-involving vs fovea-sparing detachments. Eyes with postoperative granular changes on AF tended to have worse pre- and postoperative visual outcome, and may suggest a functional deficit in certain eyes postoperatively. In this regard, AF technology is a valuable tool that aids clinicians both in preoperative characterization of the RRD and the postoperative counselling for patients with RRD.
Acknowledgments
This work was supported in part by an unrestricted educational grant to the Weill Cornell Department of Ophthalmology from Research to Prevent Blindness.
Dr Szilárd Kiss serves as a consultant to Optos PLC, the manufacturer of the ultra-wide-field autofluorescence device utilized for the current analysis. We maintained full control of the study design, collection of data, data analysis, and writing of the manuscript. Neither the company nor any of its representatives were involved in any aspect of the presented research.
References
- Afrashi F, Akkin C, Egrilmez S, Erakgun T, Mentes J. Anatomic outcome of scleral buckling surgery in primary rhegmatogenous retinal detachment. Int Ophthalmol. 2005;26 (3:77–81. doi: 10.1007/s10792-006-9004-y. [DOI] [PubMed] [Google Scholar]
- Ho CL, Chen KJ, See LC. Selection of scleral buckling for primary retinal detachment. Ophthalmologica. 2002;216 (1:33–39. doi: 10.1159/000048294. [DOI] [PubMed] [Google Scholar]
- Wilkinson CP. Retinal detachment surgery: management 25 years ago. Retina. 2006;26 (6 Suppl:S26–S27. doi: 10.1097/01.iae.0000236451.77898.d3. [DOI] [PubMed] [Google Scholar]
- Hagimura N, Iida T, Suto K, Kishi S. Persistent foveal retinal detachment after successful rhegmatogenous retinal detachment surgery. Am J Ophthalmol. 2002;133 (4:516–520. doi: 10.1016/s0002-9394(01)01427-1. [DOI] [PubMed] [Google Scholar]
- Sabates NR, Sabates FN, Sabates R, Lee KY, Ziemianski MC. Macular changes after retinal detachment surgery. Am J Ophthalmol. 1989;108 (1:22–29. doi: 10.1016/s0002-9394(14)73255-6. [DOI] [PubMed] [Google Scholar]
- Heimann HKB.Primary vitrectomy in rhegmatogenous retinal detachmentIn: Ryan S, Hinton D, Schachat A (eds)Retina4th ednElsevier Mosby: Philadelphia; 20062085–2094. [Google Scholar]
- Tani P, Robertson DM, Langworthy A. Prognosis for central vision and anatomic reattachment in rhegmatogenous retinal detachment with macula detached. Am J Ophthalmol. 1981;92 (5:611–620. doi: 10.1016/s0002-9394(14)74651-3. [DOI] [PubMed] [Google Scholar]
- Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36 (3:718–729. [PubMed] [Google Scholar]
- Lois N, Forrester J. Fundus Autofluorescence. Lippincott Williams and Wilkins: Philadelphia, PA; 2009. [Google Scholar]
- Lai WW, Leung GY, Chan CW, Yeung IY, Wong D. Simultaneous spectral domain OCT and fundus autofluorescence imaging of the macula and microperimetric correspondence after successful repair of rhegmatogenous retinal detachment. Br J Ophthalmol. 2010;94 (3:311–318. doi: 10.1136/bjo.2009.163584. [DOI] [PubMed] [Google Scholar]
- Shiragami C, Shiraga F, Yamaji H, Fukuda K, Takagishi M, Morita M.Unintentional displacement of the retina after standard vitrectomy for rhegmatogenous retinal detachment Ophthalmology 2010117(1)86–92.e1. [DOI] [PubMed] [Google Scholar]
- Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28 (3:385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- Sayanagi K, Ikuno Y, Tano Y. Different fundus autofluorescence patterns of retinoschisis and macular hole retinal detachment in high myopia. Am J Ophthalmol. 2007;144 (2:299–301. doi: 10.1016/j.ajo.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Bonnay G, Nguyen F, Meunier I, Ducasse A, Hamel C, Arndt C. Screening for retinal detachment using wide-field retinal imaging. J Fr Ophtalmol. 2011;34 (7:482–485. doi: 10.1016/j.jfo.2011.02.012. [DOI] [PubMed] [Google Scholar]