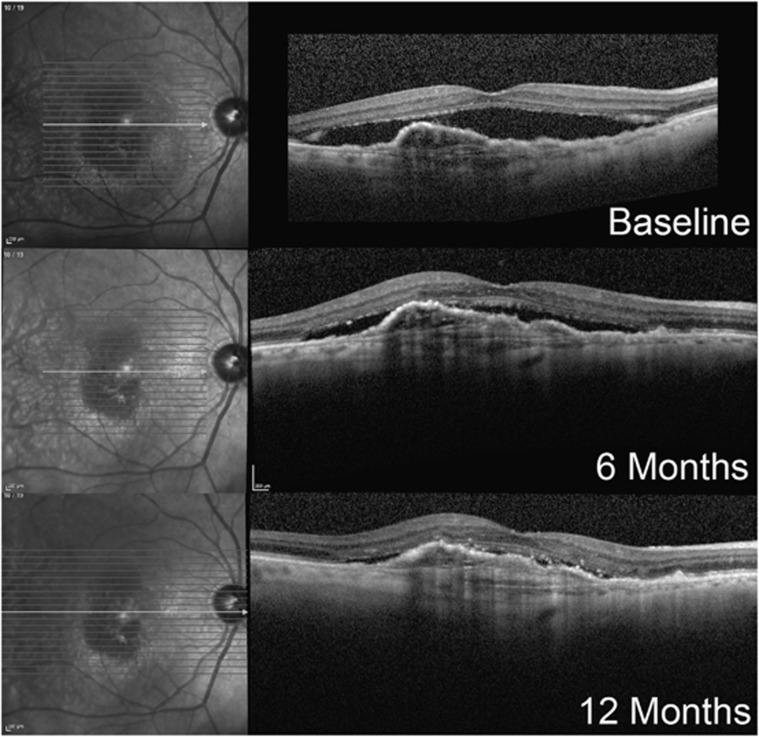
Figure 2.
Case 1 (2.0 mg Ranibizumab group). An 85-year-old male developed type 1 neovascular AMD in his right eye 3 years prior. Over this period he received 28 injections of intravitreal bevacizumab and 19 injections of intravitreal ranibizumab. The patient had been maintained on a 3-weekly alternating bevacizumab and ranibizumab dosing regimen, after SRF had been noted to recur within a standard 4-week dosing regimen. Despite this, at no time did the SRF or sub-RPE fluid completely resolve. In the year before enrolment, the BCVA had slowly declined from 20/50 to 20/80 OD. The patient received 12 intravitreal injections of ranibizumab 2.0 mg at 4-weekly intervals over the first 12 months of the trial. During this period his BCVA improved from 56 ETDRS letters at baseline to 60 letters at 6 months to 61 letters at 12 months. SRF fluid (vertical height from the top of the RPE to the IS/OS junction) at the fovea decreased from 311 μm at baseline to 109 μm at 6 months and 49 μm at 12 months. The retinal pigment epithelial detachment remained stable in size.