Table 3.
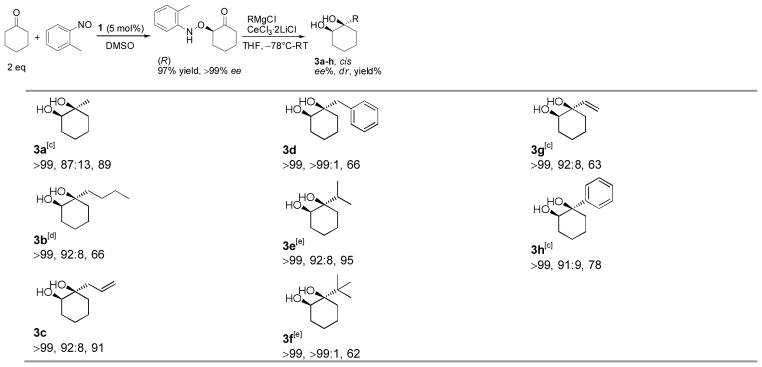
|
cis configuration and dr determined by 1H NMR.
Isolated yield of two diastereomers. Based on aminoxylated cyclohexanone.
RMgBr used in the absence of CeCl3·2LiCl.
n-BuMgCl used in the absence of CeCl3·2LiCl.
RLi used instead of RMgCl.

|
cis configuration and dr determined by 1H NMR.
Isolated yield of two diastereomers. Based on aminoxylated cyclohexanone.
RMgBr used in the absence of CeCl3·2LiCl.
n-BuMgCl used in the absence of CeCl3·2LiCl.
RLi used instead of RMgCl.