Abstract
Purpose.
To test the intra- and intersubject reproducibility of brain activation patterns that underlie visually guided saccades and word recognition in normally sighted subjects and patients with macular degeneration using functional magnetic resonance imaging (fMRI).
Methods.
Ten normally sighted subjects and five patients with macular degeneration were asked to perform two visually guided saccade tasks and two word-recognition tasks during fMRI with behavioral monitoring. The fMRI measurements were repeated three times at intervals of at least 4 weeks between sessions. The intrasubject reproducibility of the brain activation patterns was examined in a model-independent manner by comparing the distributions of activation across the frontal, parietal, temporal, and occipital brain lobes using Intraclass Correlation Coefficients (ICCs). Intersubject reproducibility was examined by repeated-measure ANOVA.
Results.
Control subjects showed overall higher intrasubject reproducibility of brain activation patterns (75% ICCs > 0.5) than that of patients with macular degeneration (56% ICCs > 0.5). The intrasubject reproducibility for the patients improved when the target location was fixed, as in the word-recognition tasks (75% ICCs > 0.5), compared with the visually saccade tasks (37% ICCs > 0.5). Intersubject variability of brain activation patterns was strikingly high for both the control and patient groups.
Conclusions.
The fMRI method can serve as a reliable within-subjects measure of brain activation that has potential for measuring longitudinal changes in brain networks associated with rehabilitation training. Striking intersubject variability reflected at the level of lobes of the brain among control subjects with similar behavioral performance, suggests individual analysis is necessary when implementing longitudinal brain activation studies.
The fMRI method can serve as a reliable within-subjects measure of brain activation that has potential for measuring longitudinal changes in brain networks associated with rehabilitation training.
Introduction
Macular degeneration is the most common visual impairment in persons over 50 years of age in the United States.1,2 The deficits in visual function as the result of macular degeneration are debilitating, because individuals lose their abilities to carry out many daily activities that require fine spatial detail recognition, such as reading.3,4 When the central visual acuity becomes progressively poorer, patients will naturally adopt one or multiple peripheral retinal loci (PRL) to substitute for the diseased fovea.5,6 The use of a PRL is often effortful and fatiguing, because it involves unnatural oculomotor control.7 Normal visual signals that correspond to a significant portion of the visual cortex associated with the macular area are disrupted. The success of some patients with macular degeneration in the successful negotiation of activities of daily life indicates that the brain is capable of compensation for this disruption.8–10 If we can understand this natural process, we may be able to enhance this process for other patients during visual rehabilitation.
Our long-term goal is to use blood oxygenation level–dependent (BOLD) functional magnetic resonance imaging (fMRI) to investigate the biological basis of such compensation mechanisms, and ultimately use this information in the design and to measure the effectiveness of visual rehabilitation programs. BOLD fMRI, characterized by high spatial resolution and noninvasiveness, is a powerful tool to investigate human brain function across distributed networks. In particular, fMRI is used to assess the cortical reorganization and brain plasticity of patients with different neural diseases.11–15 However, great variability occurs with repetition of the same fMRI studies on the same subject.16 There are a number of potential sources of this variability. The essential one may be the nature of BOLD contrast that arises from the local cerebral hemodynamic response to the increased neuronal activity associated with performing a task.17–19 BOLD contrast is small (1–5% signal change), delayed from the stimulus, and requires signal averaging with statistical methods for detection. In addition, BOLD contrast is also subject to psychologic factors, including mood, attention, strategy, and learning, as well as to nonpsychologic factors, including differences in scanner equipment, head motion, and physiologic condition. Therefore, measuring and controlling these factors are necessary for effective design and accurate interpretation of fMRI studies.
When using fMRI in patients with macular degeneration, we designed a set of paradigms to measure the changes in brain activation initially due to the disease, and then rehabilitation. The paradigms emphasized the rehabilitation strategies. Most current fMRI studies of patients with macular degeneration are focused on visual cortex reorganization.20–26 Simple visual stimuli (flashing checkerboard and pictures of natural scenes or objects) have been used in these previous studies to stimulate activation in the primary visual cortex. Beyond the shared interests of alterations in cortical activation relative to control subjects, the goal of this research has not been to focus on retinotopic mapping of the visual cortex, but instead, to investigate the brain networks that are associated with the coordination of eye movement (oculomotor control) and visual attention and recognition. These functions are highly congruent in normal-sighted people with the fovea being effortlessly placed on the point of visual attention automatically. In patients with macular degeneration, this process must be rebalanced to use a retinal location other than the fovea for successful viewing.27,28 Thus the paradigms have been designed to examine the oculomotor networks responsible for saccading the eyes to a simple moving target (dot) and visual language recognition (stationary target of increasing size from a single letter to short words and then longer words).
Four visuospatial tasks of increasing complexity have been developed with this research. The tasks encompass the oculomotor function of finding a target of variable size, or at an unpredictable location, and then the visual detection and recognition function of recognizing that target. Our battery of fMRI paradigms uses visually guided saccade tasks, similar to the function used in reading, to various targets (e.g., a moving dot, stationary one-letter, three-letter words, and six-letter words) of increasing size. These fMRI paradigms were designed to probe aspects of a reading rehabilitation program designed in our laboratory to train patients to use their PRLs more efficiently.29 All visual targets were designed at an appropriate size based on the patients' PRL sizes and visual acuity levels. Horizontal and vertical guidelines were present in the visual stimuli to help patients locate the visual target. All paradigms were designed to require subject feedback to provide performance parameters including accuracy and response time that reflect the maintained attention of the subject to the task. Previous studies in our laboratory have demonstrated that these paradigms elicit measurable brain activation in patients with age-related macular degeneration (AMD).27,28 These studies have shown that patients with AMD tend to recruit more cortical regions generally implicated in attention and effort when performing visual-saccade and word-recognition tasks.
It is essential to measure longitudinal intra- and intersubject reproducibility of brain activation patterns for these paradigms if they are to be used to investigate adaptations of brain networks in patients with macular degeneration during visual rehabilitation. Current fMRI reliability studies tend to use different indices as measures of reproducibility. These parameters include: (1) absolute activated voxel number within specific regions of interest (ROIs)30–32; (2) location of activated voxels30–32; (3) the statistical significance or contrast intensity of activated voxels33; and (4) proportion of the activated voxel within different ROIs.34–39 Proportion of the activated voxels within specific ROIs is considered to be a reliable index,40 for the sources of variability affect the whole brain in a similar fashion.41 Prior research with patients affected by stroke42 and patients with schizophrenia43 has shown greater variability in fMRI data for patients than for control subjects. However, moderate or even good reproducibility has been achieved among the patients with stroke when they perform a drawing task using the less affected hand.44
In the present study, we report on the reproducibility of performance and brain activation patterns of normally sighted adults and patients with macular degeneration during the previously described visuospatial tasks. Unlike most other fMRI studies, we did not use specific ROI analysis based on a known network model. Such an approach may obscure different strategies used by different subjects to achieve success on any given visual task or changing strategies by a single subject on a repeated task. Instead, a model-independent analysis using the lobes of the brain was used to characterize the variability of global activation patterns. By separating the brain at the lobe level, any variability on this scale can be thought of as reflecting distinctly different cognitive patterns across sessions and across subjects. If present, it would reflect widely changing cognitive strategies. High variability in individual subjects would suggest a less consistent cognitive strategy. High variability among subjects would suggest different cognitive strategies.
Methods
Participants
The demographic data of the control subjects (n = 10) and patients (n = 5) are found in Table 1. The control subjects consisted of younger normally sighted control subjects (n = 5, subjects 100–104, mean visual acuity [VA] = 20/18.4 ± 3.6) and older normally sighted control subjects (n = 5, subjects 200–204, VA = 20/21.6 ± 2.2). The patients consisted of those with the juvenile-onset macular degeneration (Stargardt disease, n = 2, subjects 300, 301) and those with AMD (n = 3, subjects 400–402). For all patients, the VA (for the better eye) was equal to 20/189.8 ± 161.0. For each patient, the currently used PRL was assessed by our acuity microperimetry system developed in our laboratory29 and a microperimeter (MP-1; Nidek Technologies, Tokyo, Japan) with eye-tracking control capabilities. The PRL sizes and locations are also listed in Table 1. Signed informed consent, approved by Institutional Review Board of The University of Illinois, was obtained from each subject prior to the study.
Table 1. .
Demographic Information for the Participants
|
Case Number |
Age (y) |
Sex |
Visual Acuity (VA) |
PRL Size* (degrees) |
|
|
OD |
OS |
||||
| Controls | |||||
| 100 | 27 | M | 20/18 | 20/15 | |
| 101 | 22 | M | 20/17 | 20/16 | |
| 102 | 33 | F | 20/20 | 20/17 | |
| 103 | 23 | F | 20/20 | 20/20 | |
| 104 | 48 | M | 20/24 | 20/24 | NA |
| 200 | 70 | M | 20/21 | 20/20 | |
| 201 | 63 | M | 20/25 | 20/24 | |
| 202 | 61 | M | 20/22 | 20/20 | |
| 203 | 54 | F | 20/24 | 20/24 | |
| 204 | 65 | F | 20/20 | 20/20 | |
| Patients | |||||
| 300 | 44 | F | 20/60 | 20/32 | 12 (OS) |
| 301 | 55 | F | 20/400 | 20/450 | 9 (OD) |
| 400 | 64 | M | 20/76 | 20/91 | 12 (OD) |
| 401 | 77 | F | 20/121 | 20/166 | 6 (OD) |
| 402 | 82 | F | 20/320 | 20/560 | 3 (OD) |
100 to 104: Younger normally sighted control subjects; 200 to 204: Older normally sighted control subjects; 300 to 301: Patients with macular degeneration due to Stargardt disease; 400 to 402: Patients with age-related macular degeneration. PRL, Preferred Retinal Locus; NA, not applicable.
* The PRL size for the better eye is shown.
fMRI Paradigms
The four tasks are illustrated in Figure 1. All paradigms used the same block design consisting of six cycles; each cycle was comprised of a rest period of 30 seconds followed by a stimulus period of 30 seconds. All paradigms began with 12 seconds of discarded data acquisition (to ensure equilibrium of the longitudinal magnetization) and ended with a 30-second stimulus condition. Orthogonal vertical and horizontal guidelines with a width of 0.4° were provided to help patients locate the targets in each paradigm. The size of each single target (a dot or single letter) was 2°, which was within the sizes of the PRL of all patients (Table 1). Thus, each patient had the potential to capture the complete single target in the dot and letter paradigms, and a single letter in the word-recognition paradigm. All subjects and patients were studied with all paradigms in three separate sessions, each separated by approximately 4 weeks. The total interval covered by three sessions was around 8 weeks. The 8-week interval reflected the length of our reading rehabilitation program.
Figure 1. .
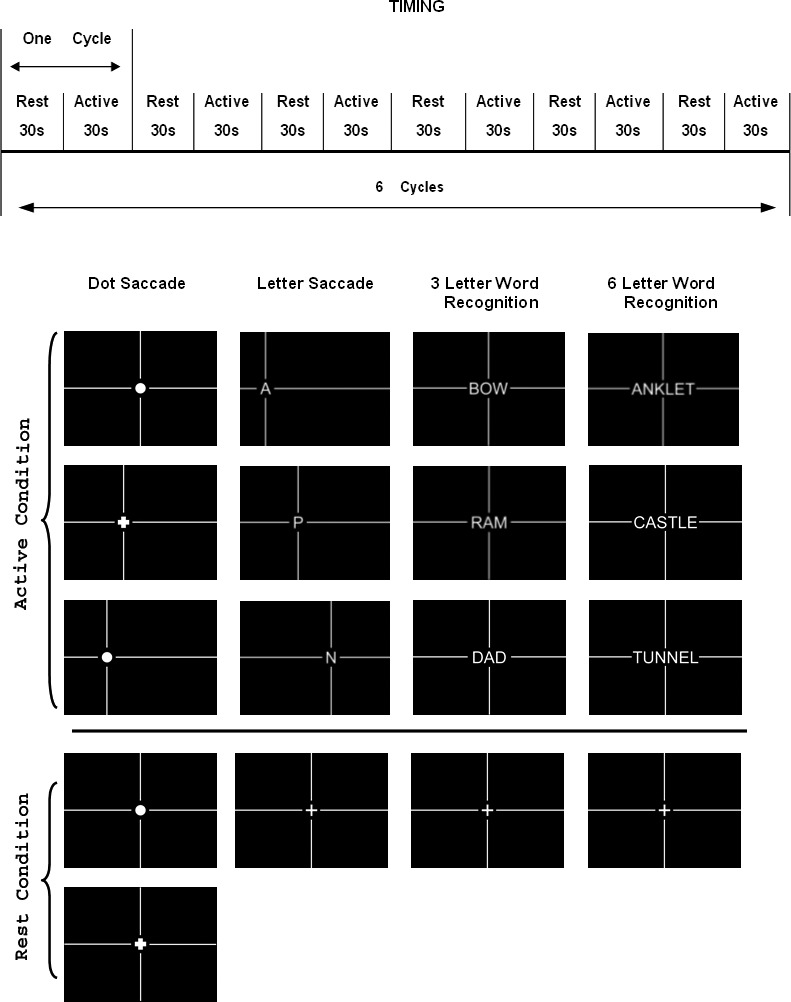
Schematic of the timing diagram of the fMRI protocol and examples of the visual stimulus patterns of the 4 paradigms.
Visually Guided Dot Saccade Task (DS).
The DS task required the subject to locate a dot (subtending 2° of visual angle, equivalent to a 20/600 stimulus) that was presented in one of seven possible unpredictable locations (at 3°, 6°, or 9° on either side of a center 0° location). Every 1.5 seconds, the position of this dot changed to the position immediately 3° to either the left, or right, of the previous position. The subject was required to indicate by pressing a response button when the dot changed to a cross (also subtending 2°). The rest condition for the DS task required maintained fixation on a 2° dot located at the center of the screen. In addition, the subjects were asked to respond by pressing a button when the dot became a cross. The finger switch responses were designed to maintain and measure attention during the paradigm.
Letter Saccade Task (LS).
The LS task was the same basic task as the DS task except a 2° letter, that changed unpredictably, was substituted for the dot and a finger switch response was required when the letter presented was a vowel. The rest condition for the LS task required maintained fixation on a 2° cross located at the center of the screen. The finger switch responses were designed to measure not only attention but also demonstrate recognition of the target indicating that functional retina and presumably the PRL was being used for the task
Three-Letter and Six-Letter Word-Recognition Tasks (3L and 6L).
For this task, either a three-letter or a six-letter word was presented at the center of the screen. Subjects were asked to press one switch if the word represented a living thing, and the other switch if the word represented a nonliving thing. During the stimulus period, the word presented was changed every 3 seconds. Each letter subtended 2°. The rest condition required maintained fixation on a 2° cross that was located at the center of the screen. The use of a word simplified the planning of the eye movements because, once the first letter was found, the eye movements were always rightward as in reading. The longer word potentially required more eye movements, although word recognition/perceptual filling-in also plays an important role without requiring additional eye movements.
Data Collection
Eye Movement Data Collection in the Low Vision Laboratory.
Eight control subjects (100–102, 104, 200–203) and all patients were trained prior to scanning on the four tasks outside of the scanner in our behavioral laboratory, and their eye movements during these practice sessions were recorded using an eye tracker that tracks the pupil and corneal reflections (Model 504; Applied Sciences Laboratory, Bedford, MA). The sampling and output rate of the eye tracker system was 60 Hz. During training, the subjects were seated at a viewing distance of 40 cm from the high-resolution display monitor (1024 × 768 pixels). Their head position was maintained by a forehead and chin support.
Imaging Data Collection.
A 3.0-Tesla whole body scanner (EXCITE 2.0; GE Healthcare, Waukesha, WI) using serial gradient echo, echo-planar imaging (plane = axial, repetition time [TR] = 2999 ms, time to echo [TE] = 25 ms, flip angle [FA] = 90°, bandwidth = 62 kHz, voxel size = 3.125 mm × 3.125 mm × 3 mm; acquisition matrix = 64 × 64, field of view [FOV] = 20 × 20 cm2, slice thickness/gap = 3/1 mm/mm, slices = 34) was used for all image acquisitions. The paradigms were presented in the scanner projected onto a visor and coordinated with behavioral and physiological measurements (MRIx Technologies, Bannockburn, IL). When the subjects performed the tasks in the scanner, their eye movements were monitored within the scanner to ensure that every subject was performing the appropriate eye movements for the task (MRIx Technologies). Head position was stabilized with a tightly fitting head pillow in the volume radiofrequency coil.
Following the completion of each functional imaging session, a single 3D high-resolution anatomical scan was acquired (3D inversion recovery fast spoiled gradient recalled echo sequence, plane = axial, TR = 9ms, TE = 2.0 ms, FA = 25°, bandwidth = 15.6 kHz, acquisition matrix = 256 × 256, FOV = 22 × 16.5 cm2, slice thickness/gap = 1.5/0 mm/mm, slices = 124).
Data Analysis
Eye Movement Data Analysis.
The subjects' eye movements, recorded outside the scanner, included x (horizontal) and y (vertical) coordinates of gaze and pupil diameter at each temporal sampling point. A blink was represented by a zero pupil diameter for a certain time period. The gaze positions from 0.04 second before a blink to 0.2 second after a blink were distorted, therefore were excluded from further quantification. In the present study, eye movement data within one representative cycle of each paradigm is presented. The eye movement pattern in the fixation condition was characterized by the gaze standard deviation (SD) during a complete block (30 seconds) of fixation in both the x (SD_fixation_x) and the y (SD_fixation_y) directions. The eye movement pattern in the task condition was characterized by averaging the gaze SD during the late 2/3 time period of each stimulus trial in the x (Mean_SD_trial_x) and the y (Mean_SD_trial_y) directions. This is the period after the target has been found and is maintained presumably on the PRL.
The gaze locations within the beginning 1/3 time period of each trial were excluded because the saccadic eye movement used to relocate gaze on the new target during this period increased the gaze SD that was a measure of fixation ability. The Mann–Whitney test was used to compare the eye movement patterns between the control group and the patient group. All the analyses above were done using customized software (Matlab; The MathWorks, Inc., Natick, MA).
fMRI Data Analysis.
A locally developed software package (NIVANA; MRIx Technologies) and the software package AFNI45 were used to analyze the fMRI data. Data from any imaging session with head movement exceeding 1 mm (1/3 of a voxel dimension) were excluded from further analysis. This accounts for the data that are indicated as missing in the Results section. Overall, 16 of 150 (10.7%) acquisitions have been excluded for control subjects, and 17 of 75 (22.7%) acquisitions have been excluded for patients.
Brain activation maps were produced by comparing the voxel signal intensity between task condition and rest condition via a voxelwise two-tailed Student's t-test. Thresholds of different t-values were examined. Consistency does not increase with different thresholds, until a very high threshold is tested, when only the primary visual cortex is active and there are too few voxels to be meaningful. A lower threshold that includes the eye movement network known to operate in these tasks is appropriate for investigating consistency of responses across individuals. The data presented are for a uniform t-value equal to 3 for all subjects. Subsequently, individual functional activation maps for each paradigm were superimposed over the structural images from the same individual.
Activation patterns, characterized as the percentages of activated voxels in each lobe (frontal, parietal, temporal, and occipital) of the brain collapsed across hemispheres, were used to determine the fMRI reproducibility across sessions and subjects. Intraclass correlations were applied to test for the significant intrasubject variations of the activation patterns and the performance data for each paradigm across sessions. In addition, repeated-measure ANOVA was used to investigate the intersubject variability for each paradigm and session.
Percentage of Voxel Activation
The lobes of the cerebrum, (frontal, parietal, temporal, and occipital) were defined according to sulcal landmarks using each individual subject's anatomic images, and defined by the neuroradiologist on our team (KRT). The frontal lobe was defined as the tissue anterior to the central sulcus and superior to the Sylvian fissure (lateral sulcus). The parietal lobe was defined as the tissue posterior to the central sulcus, medial to the Sylvian fissure and superior to parietooccipital fissure. The temporal lobe was defined as the brain parenchyma lateral to the Sylvian fissure and anterior to the lateral projection of the parietooccipital sulcus. The occipital lobe was defined as all tissue posterior and inferior to the parietooccipital fissure and above the tentorium.
The number of activated voxels for each lobe (Vi) was counted and calculated as a percentage (Pi) of the total number of activated voxels in all four lobes of the cerebrum (Vt):
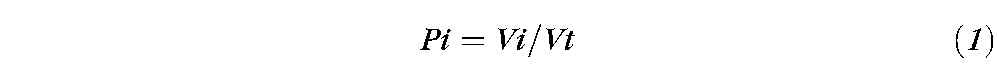 |
The percentage activation in each lobe was then displayed in a pie chart to summarize the activation patterns of each paradigm for each subject for each session.
Intraclass Correlation Coefficient
The Intraclass Correlation Coefficient (ICC) is well known in psychometry as an index of reliability46 and has been applied to determine the reliability of the fMRI activation patterns. There are several ICC model alternatives, and this study uses the one-way ANOVA model (ICC1) that is specifically suitable for this type of fMRI data.31,33 In this study, only within-subject variance (σwithin) and between-subject variance (σbw)) are considered. ICC estimates the proportion of variance that is due to differences between the subjects rather than differences due to the measurements; therefore, it can be described by the following equations 2 and 3. Thus, smaller values of σwithin result in a higher ICC.
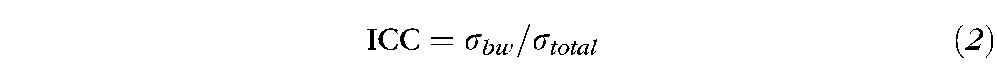 |
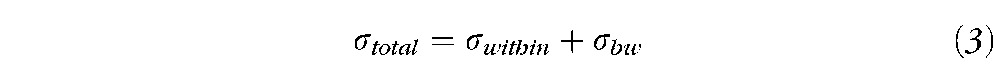 |
From a computational view, ICC, σbw and σwithin are represented by the mean square between n subjects (MSB) and mean square within subjects (MSW). In equations 4 and 5, index i represents ith session, and index j represents the jth subject. The symbol k is the number of total sessions, which is equal to 3. The total subject number, n, is different in different paradigms due to data that were excluded due to head movement. We applied the ICC analysis on the activation percentage for each lobe of the brain for each paradigm across the three sessions.
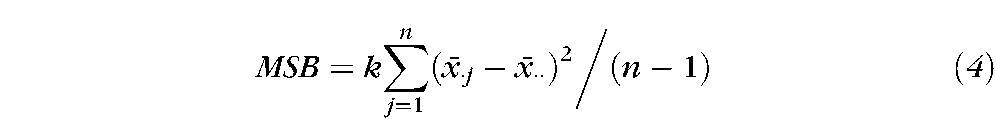 |
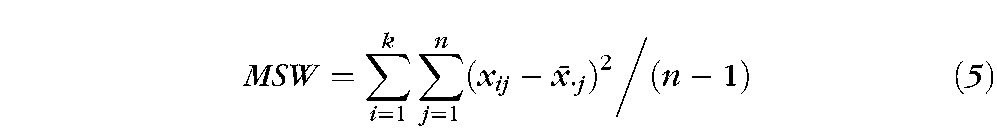 |
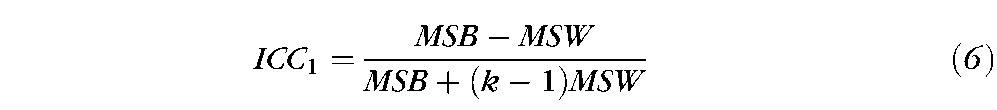 |
Results
Eye Movement Data
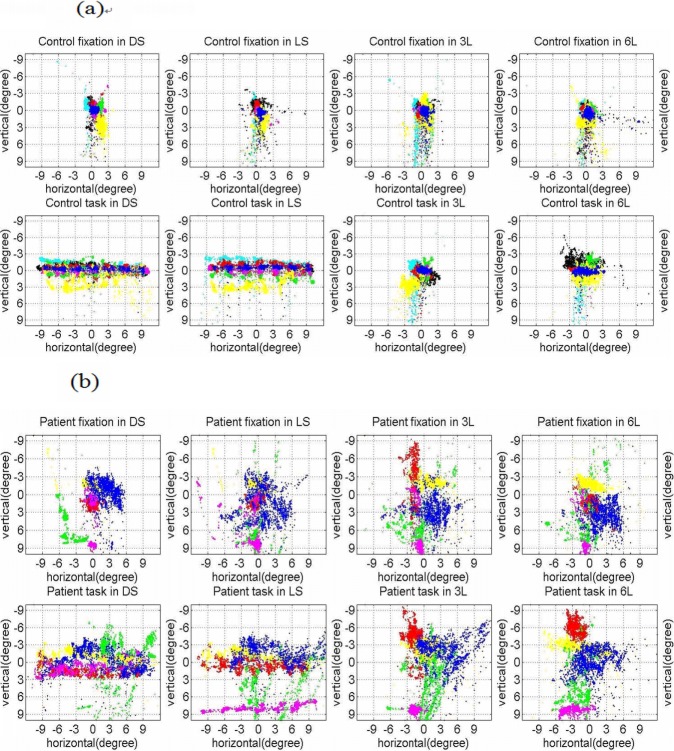
Figure 2 shows the eye movement patterns collected in the behavioral laboratory for the eight control subjects and the five patients during fixation (upper row in each pair) and the active condition (lower row in each pair) for each of the four paradigms. Table 2 is a quantitative summary of these eye movement patterns for these same subjects and patients. The eye movement pattern was quantified by the previously defined variables: SD_fixation_x, SD_fixation_y, Mean_SD_trial_x, Mean_SD_trial_y. The results of the Mann–Whitney test used to compare these variables between the eight control subjects and the five patients is indicated in Table 2. From Figure 2 and Table 2, the patients show greater variation in fixation and an increased variation in saccades to all types of targets compared with the control subjects. However, it is also evident that all of the participants were able to perform the tasks of moving their eyes to targets across the visual field.
Figure 2. .
Eye movement patterns for (a) control subjects (n = 8) and (b) patients (n = 5) in all 4 paradigms. The upper and lower rows in (a) and (b) show the eye movement patterns in the fixation condition and during the active condition of each paradigm for the controls and patients, respectively. All eye movement data are ordered by task; dot saccade (DS), letter saccade (LS), three-letter word recognition (3L), and six-letter word recognition (6L) from left to right. The colors indicate the patterns for individual subjects. Control subjects: 100 = red, 101 = green, 102 = cyan, 104 = magenta, 200 = yellow, 201 = black, 202 = red in small circle, 203 = blue. Patients: 300 = red, 301 = green, 400 = magenta, 401 = yellow, 402 = blue.
Table 2. .
Quantification of Eye Movement Patterns for Four Paradigms
|
|
n |
DS |
LS |
3L |
6L |
|
| Gaze SD during fixation condition in degrees | ||||||
| SD_fixation_x | Control | 8 | 0.42 ± 0.17 | 0.59 ± 0.16 | 0.62 ± 0.38 | 0.42 ± 0.19 |
| Patient | 5 | 1.37 ± 1.02* | 1.51 ± 0.99* | 1.66 ± 1.28 | 1.77 ± 0.64** | |
| SD_fixation_y | Control | 8 | 0.84 ± 0.52 | 1.25 ± 0.70 | 1.26 ± 0.70 | 0.93 ± 0.34 |
| Patient | 5 | 1.70 ± 1.32 | 2.05 ± 1.16 | 2.52 ± 1.36* | 2.68 ± 1.19** | |
| Averaged gaze SD during each stimulus trial in task condition in degrees | ||||||
| Mean_SD_trial_x | Control | 8 | 0.35 ± 0.09 | 0.35 ± 0.16 | 0.44 ± 0.22 | 0.75 ± 0.51 |
| Patient | 5 | 0.77 ± 0.34** | 1.02 ± 0.50** | 1.26 ± 0.82* | 1.60 ± 0.83* | |
| Mean_SD_trial_y | Control | 8 | 0.20 ± 0.07 | 0.32 ± 0.20 | 0.71 ± 0.57 | 0.63 ± 0.37 |
| Patient | 5 | 0.82 ± 0.61** | 1.09 ± 0.98 | 1.61 ± 1.01 | 1.20 ± 0.61 | |
SD_fixation_x and SD_fixation_y represent the SD of the gaze fixation position in the x (horizontal) direction and the y (vertical) direction, respectively, during the 30-second fixation condition. Mean_SD_trial_x and Mean_SD_trial_y represent the SD of the average gaze position in the x direction and y direction, respectively, during the late 2/3 time period of each stimulus trial in each paradigm. Statistical significance for comparisons of control and patient groups is indicated by asterisks.
* P < 0.05.
** P < 0.01.
Performance Data
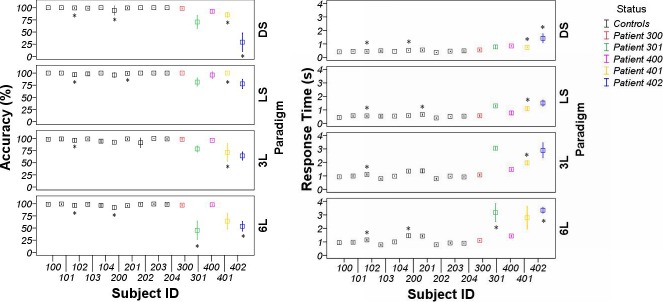
Figure 3 illustrates the performance data during fMRI for all paradigms, including task accuracy and response time. The error bars represent the SD values of accuracy and response time across the three sessions. The patients have significantly lower mean accuracy and longer mean response time across sessions than the control subjects (P < 0.05) for all four paradigms.
Figure 3. .
Error bar graph illustrating subjects' accuracy (left panels) and response time (right panels) during fMRI using the dot saccade (DS), letter saccade (LS), three-letter word recognition (3L), and six-letter word recognition (6L) paradigms. Error bar represents 1 SD of accuracy and response time across the 3 sessions. Each patient's data are represented by the same color used in Figure 2.
Table 3 is a numerical summary of the mean accuracy and response times across the three sessions, as well as the SD of accuracy and response time across the three sessions for both the control and the patient groups in each paradigm. From both Figure 3 and Table 3, we observed that all control subjects achieved a similar performance level, whereas there was great intersubject variability for performance within the patient group. Variance ratio test (F test) results confirmed our observation as indicated in Table 3. Furthermore, the control subjects tended to achieve more consistent intrasubject behavioral patterns compared with those of the patients. This tendency is reflected by the lower SD values of accuracy and response time across sessions in all paradigms for the control group. However, the Mann–Whitney test that was used to compare the SD of accuracy and response times across sessions between the control and patient groups did not reach general significance (the only statistically significant result was for the DS paradigm), probably because of the small sample size. The intrasubject variability of the patients on performance appeared to be closely related to their visual acuity and PRL size. Patient 300 and Patient 400 who achieved relatively consistent performance across sessions had better visual acuity (VA Patient 300 = 20/32, VA Patient 400 = 20/76) and larger PRL sizes (12°) compared with that of the other three patients.
Table 3. .
Numerical Summary of fMRI Performance Data
|
|
DS |
LS |
3L |
6L |
||||
|
Control |
Patient |
Control |
Patient |
Control |
Patient |
Control |
Patient |
|
| Mean Acc across sessions (%) | 99.3 ± 1.7 | 75.4 ± 27.8** | 99.0 ± 1.5 | 91.1 ± 10.6** | 96.8 ± 3.2 | 81.7 ± 15.1** | 97.6 ± 2.27 | 71.7 ± 24.5** |
| SD of Acc across sessions (%) | 1.2 ± 2.9 | 8.4 ± 7.6 | 1.3 ± 1.8 | 5 ± 4.7 | 2.4 ± 2.7 | 7.0 ± 6.8 | 2.1 ± 1.1 | 9.8 ± 7.9 |
| Mean RT across sessions (s) | 0.5 ± 0.05 | 0.9 ± 0.33** | 0.5 ± 0.07 | 1 ± 0.38** | 1 ± 0.20 | 2.1 ± 0.86* | 1 ± 0.24 | 2.4 ± 1.03* |
| SD of RT across sessions (s) | 0 ± 0.02 | 0.1 ± 0.13* | 0 ± 0.02 | 0.1 ± 0.07 | 0.1 ± 0.04 | 0.2 ± 0.21 | 0.1 ± 0.04 | 0.4 ± 0.37 |
A variance ratio test (F test) was used to compare the variance of mean Acc and mean RT across sessions between control group and patient group. A Mann–Whitney test was used to compare the SD of accuracy and response time across sessions between the control and patient groups. Statistical significance is indicated by asterisks. Acc = accuracy; RT = response time; DS = dot saccade; LS = letter saccade; 3L = 3-letter word recognition; 6L = 6-letter word recognition.
* P < 0.05.
** P < 0.01.
Pie Chart Matrix and ICC Summary
Figures 4 and 5 contain the pie charts illustrating the distribution of activation across the brain for each control subject and patient, respectively, for each task paradigm, accompanied by the ICC results. Visual inspection of these pie chart matrices indicates two major points. First, there is relative consistency of distribution patterns across sessions (along columns) for each paradigm for both the control subjects and patients, with the control subjects having higher consistency than that of patients. Second, a review of the patterns across subjects and patients (along rows), in Figures 4 and 5, shows striking variability among members of both the control and patient groups.
Figure 4. .
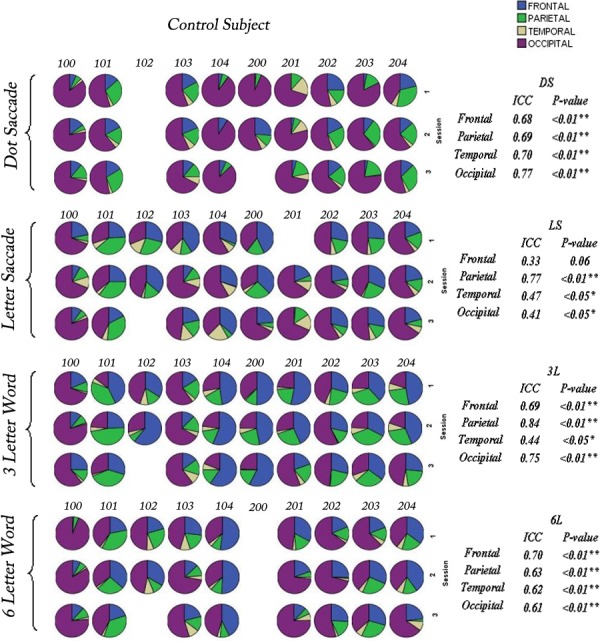
Pie charts for control subjects (100–204) illustrating the percentage activation across the 4 lobes of the cerebrum: frontal lobe = blue, parietal lobe = green, temporal lobe = yellow, and occipital lobe = magenta for all control subjects across 3 sessions for the 4 paradigms. Intraclass correlation has been applied to quantify the reproducibility of the activation patterns for each lobe across 3 sessions for the 4 paradigms. Intraclass correlation coefficients (ICCs) are presented on the right text panel. Significant correlations are indicated by asterisks (*P < 0.05; **P < 0.01). The missing pie charts indicate where data were not calculated due to excessive head movement. Overall, 16 of 150 (10.7%) acquisitions have been excluded from the control subject group.
Figure 5. .
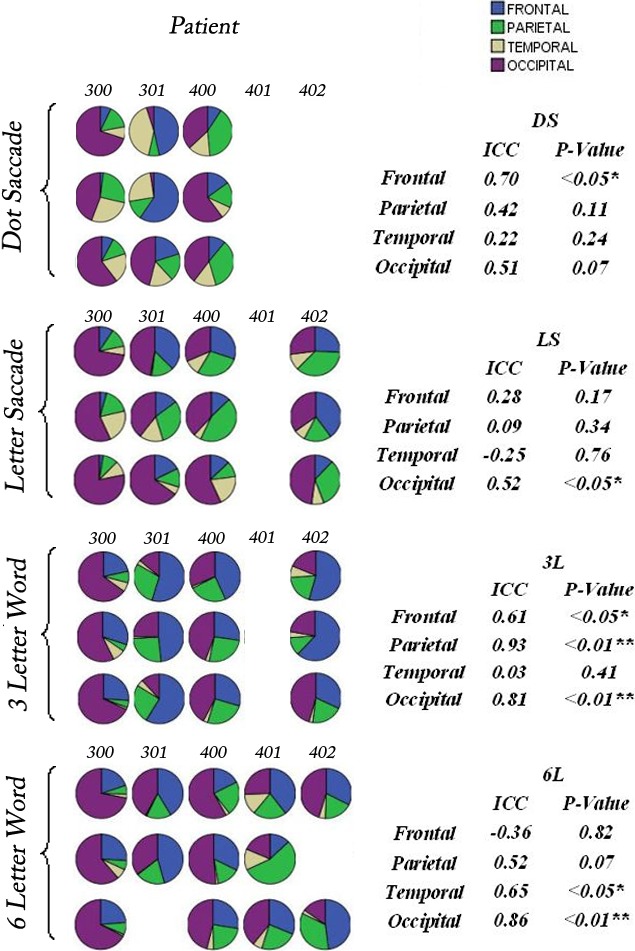
Pie charts for the patients (300–402) illustrating the percentage activation across the 4 lobes of the cerebrum: frontal lobe = blue, parietal lobe = green, temporal lobe = yellow, and occipital lobe = magenta for all patients across sessions for the 4 paradigms. Intraclass correlation has been applied to quantify the reproducibility of activation patterns for each lobe across 3 sessions for the 4 paradigms. ICCs are presented on the right text panel. Significant correlations are indicated by asterisks (*P < 0.05; **P < 0.01). The missing pie charts indicate that data were not calculated due to excessive head movement. Overall, 14 of 60 (23%) acquisitions have been excluded for the patient group.
For control subjects, 12 of 16 (75%) ICCs > 0.5 and 15 of 16 (94%) correlation coefficients were significant, indicating strong intrasession consistency. For patients, 9 of 16 (53%) ICCs > 0.5 and 7 of 16 (44%) correlation coefficients were significant. The intrasubject consistency for patients improved when the target location was fixed, as in the word-recognition tasks. The patients showed 6 of 8 (75%) ICCs > 0.5 and 5 of 8 (63%) significant ICCs in 3L and 6L paradigms. Considering the statistical significance of ICCs is skewed by a smaller sample size of the patient group, we use the absolute ICC value to represent intrasubject reproducibility of the brain activation patterns for control subjects and the patients. Overall, patients showed reasonably good reproducibility in word-recognition tasks. The reproducibility of activation within each lobe across sessions for each paradigm was demonstrated for the control subjects and patients. This lobar region of interest analysis permitted patterns to be compared without reference to specific networks that appear to have large variations among individuals, although being reproducible across sessions for individuals. Representative brain activations for a control subject (203) and a patient with Stargardt disease (300) across three sessions for each paradigm are shown in Figures 6 and 7, respectively. The maps display consistent areas of activation across the sessions in regions of the brain associated with eye movement, right hand sensorimotor movement (finger switch), and reading, including the frontal eye fields, supplementary eye fields, prefrontal cortex, intraparietal sulcus, and the visual cortex (V1, V2/V3, MT/V5).
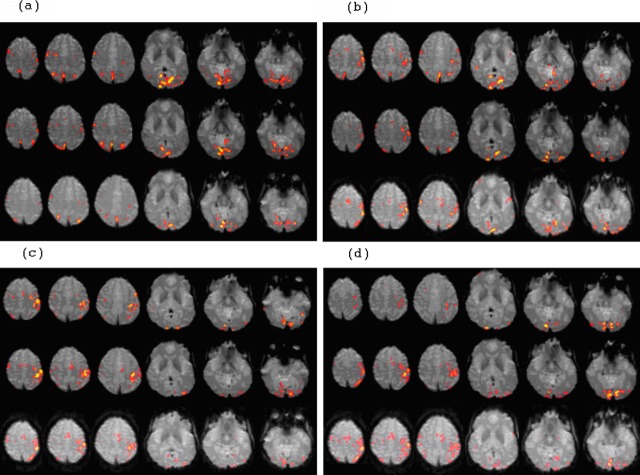
Figure 6. .
Representative activation patterns for an older control subject (203) across each of 3 scan sessions (top, first session; middle, second session; bottom, third session) for each of the 4 paradigms: (a) dot saccade paradigm, (b) single-letter saccade paradigm, (c) three-letter word paradigm, and (d) six-letter word paradigm. Head motion was similar in each session and less than 33% of a voxel dimension (3 mm isotropic). Performances on recognition tasks were better than 90% (Fig. 3).
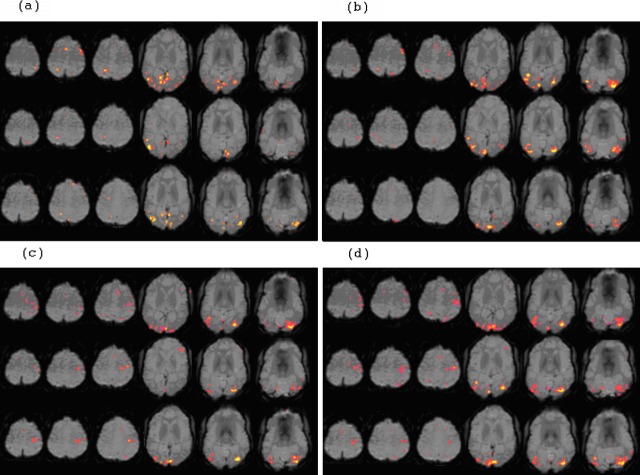
Figure 7. .
Representative activation patterns for a patient (300) across each of 3 scan sessions (top, first session; middle, second session; bottom, third session) for each of the 4 paradigms: (a) dot saccade paradigm, (b) single-letter saccade paradigm, (c) three-letter word paradigm, and (d) six-letter word paradigm. Head motion was similar in each session and less than 33% of a voxel dimension (3 mm isotropic). Performances on recognition tasks were better than 90% (Fig. 3).
Intersubject Variability
The repeated-measure ANOVA, used to quantify the intersubject variability for the activation patterns for the control subjects and the patients, is summarized in Table 4. For the control subjects, statistical significance was achieved for 16 of 16 F values for a between-subjects effect, indicating substantial intersubject variability. By comparison, none of the F values for the within-subjects effect (Session factor) showed statistical significance, indicating little variability across sessions. This is consistent with the ICC results for the control subjects. For the patients, statistical significance was achieved for 11 of 16 F values for the between-subjects effect. Only 1 of the 16 F values for the within-subjects effect showed statistical significance. This result indicates substantially larger brain activation variability for the Subject factor than for the Session factor among the patients.
Table 4. .
Intersubject and Intersession Variability from a Two-Way ANOVA of Brain Activation Patterns for Four Paradigms
|
Paradigm |
Lobe |
Control Group |
Patient Group |
||||||
|
Within-Subject |
Between-Subject |
Within-Subject |
Between-Subject |
||||||
|
F Value |
P Value |
F Value |
P Value |
F Value |
P Value |
F Value |
P Value |
||
| Dot saccade paradigm | Frontal | 1.36 | 0.29 | 28.42 | <0.01** | 0.80 | 0.51 | 3.10 | 0.22 |
| Parietal | 0.95 | 0.41 | 34.54 | <0.01** | 0.05 | 0.95 | 15.17 | 0.06 | |
| Temporal | 1.48 | 0.26 | 10.49 | <0.05* | 0.12 | 0.89 | 19.64 | <0.05* | |
| Occipital | 0.09 | 0.91 | 214.16 | <0.01** | 0.39 | 0.70 | 11.53 | 0.08 | |
| Letter saccade paradigm | Frontal | 1.77 | 0.21 | 106.95 | <0.01** | 2.44 | 0.17 | 15.91 | <0.05* |
| Parietal | 1.77 | 0.21 | 18.47 | <0.01** | 1.01 | 0.42 | 35.70 | <0.01** | |
| Temporal | 1.67 | 0.22 | 24.79 | <0.01** | 0.61 | 0.58 | 62.02 | <0.01** | |
| Occipital | 0.95 | 0.41 | 193.15 | <0.01** | 12.05 | <0.01** | 48.87 | <0.01** | |
| 3-Letter word paradigm | Frontal | 1.08 | 0.36 | 60.79 | <0.01** | 0.50 | 0.63 | 37.85 | <0.01** |
| Parietal | 1.39 | 0.28 | 51.26 | <0.01** | 1.30 | 0.34 | 14.96 | <0.05* | |
| Temporal | 0.06 | 0.94 | 41.65 | <0.01** | 0.47 | 0.64 | 27.99 | <0.05* | |
| Occipital | 0.87 | 0.44 | 42.90 | <0.01** | 1.13 | 0.38 | 14.03 | <0.05* | |
| 6-Letter word paradigm | Frontal | 0.84 | 0.45 | 37.78 | <0.01** | 0.09 | 0.92 | 348.36 | <0.01** |
| Parietal | 0.80 | 0.47 | 23.89 | <0.01** | 0.44 | 0.67 | 6.60 | 0.12 | |
| Temporal | 3.65 | 0.053 | 24.23 | <0.01** | 0.95 | 0.46 | 4.69 | 0.16 | |
| Occipital | 0.11 | 0.90 | 100.23 | <0.01** | 1.04 | 0.43 | 18.38 | <0.05* | |
Session and subject are treated as two random factors. Significant correlations are indicated by asterisks.
* P < 0.05.
** P < 0.01.
Discussion
We have examined the brain activation reproducibility for normally sighted control subjects and patients with macular degeneration using paradigms and performance measures that test visual ability to find a target, identify that target, and then maintain fixation on that target. These are the skills required for reading and are trained during our visual rehabilitation program designed to improve reading skills in patients with AMD. Our goal was to establish if fMRI can assess the biological basis of this training program. This goal requires that there be reproducible activation patterns over time for a stable skill strategy that would be expected in normal-sighted individuals. As patients with compromised vision learn new strategies for coping with their deficit, variable activation patterns may be expected and activation patterns may change as skill strategies change. To allow for such variability and changes in patterns in the analysis of the fMRI data, it was important to avoid imposing a model on these activation patterns. The use of the proportions of activation in a lobar pattern avoided any assumptions about structural underpinnings of any functional networks to reflect global activation patterns. This activated voxel proportions approach within the four brain lobes provided a large-scale measure of activation patterns. Any variability on this large regional scale can be thought of reflecting distinctly different cognitive patterns across sessions and across subjects.
Intrasubject Reproducibility
Although data from both normally sighted control subjects and visually compromised patients show statistically significant intrasubject consistency, the control subjects show higher consistency than that of patients with macular degeneration, as would be expected given their better performance. This result is consistent with previous studies on fMRI reliability cited earlier that were involved with patients affected by stroke42 and schizophrenia.43
Given the criterion that ICC > 0.5 indicates good reproducibility,43 patients with macular degeneration show reasonably good reproducibility in word-recognition tasks when the target location was fixed (6 of 8 ICCs > 0.5, ranging from 0.61–0.93). However, with moving targets in the visually guided saccade paradigms, the patients showed greater variability in brain activation pattern (3 of 8 ICCs > 0.5, ranging from 0.51–0.70). This may reflect reduced skill or variable strategies being used to find the target. Across four paradigms, the intrasubject reproducibility was highest for the occipital lobe (4 of 4 ICCs > 0.5, ranging from 0.51–0.86). Because the activation in the occipital lobe relates to visual processing, rather than the oculomotor pathways of the frontal and parietal lobes used to direct the eyes and presumably the PRL of the retina to the target, these results suggest the major challenge for patients with macular degeneration may be oculomotor control. Unlike normally sighted individuals with congruent gaze and attention at the fovea, the patients with AMD must retrain the oculomotor control network to place the PRL rather than the destroyed fovea on the target. This increased difficulty of eccentric viewing may explain why the patients achieved more stable brain activation patterns across sessions for paradigms with a fixed target with fewer eye movements as in the 3L paradigm. As demonstrated by our previous studies, patients tended to recruit more higher-order cortical regions, such as prefrontal cortex in the frontal lobe, and intraparietal sulcus in the parietal lobe, to compensate for their compromised visual system.27,28 However, it may be difficult for them to naturally develop a stable strategy to efficiently control eye movement for eccentric viewing. This unstable high-order compensation strategy may lead to the larger intrasubject variability for the frontal lobe and the parietal lobe compared with the occipital lobe for patients with macular degeneration. Rehabilitation training may help patients to establish more efficient eccentric viewing strategies for reading by achieving better oculomotor control using a PRL. The success of training may be reflected by a reduction in variability of brain activation patterns for visually guided saccade paradigms.
Compared with other patients studies using a similar statistical analysis method,43,44 we achieved a higher intrasubject reproducibility than the results obtained by Manoach et al.,43 which showed low intrasubject ICCs for the patients with schizophrenia (1 of 6 ICCs > 0.5) in all 6 ROIs using percentage signal change in the voxel with the maximum t statistic as the activation index. We achieved a similar but slightly lower intrasubject reproducibility level than did Kimberley et al.,44 who reported ICCs ranging from 0.52 to 0.94 across 6 ROIs obtained from six stroke patients using average percentage signal change within each ROI as the measurement index. The variability among these results may largely come from the brain activation index used to measure reproducibility. Manoach et al.43 sampled the percentage signal intensity change in only 1 voxel for each ROI, and Kimberley et al.44 sampled the average percentage signal intensity change across all voxels within each ROI. In the present study, we considered all activated voxels in the whole brain and used the activation proportion in each lobe as an activation index. The results demonstrated that whereas the activation level of a single voxel may differ substantially across sessions in patients with a neural deficit, the general activation index extracted from a larger amount of voxel data may have higher reproducibility. Care must be taken when comparing activation patterns across different patient populations where the disease affects the brain directly (e.g., stroke, schizophrenia) and where the disease affects the input into the brain (e.g., AMD). The compensation mechanisms and the rehabilitation potential may be quite different.
Intersubject Reproducibility
The striking intersubject variability of brain activation pattern is found in both the control and patient groups. This is consistent with the findings of previous reproducibility studies.47,48 This intersubject brain activation pattern variability at the lobe level must be considered when we try to generate multisubject level inferences for these paradigms. Currently, multisubject analysis falls into two types. One is a fixed-effects model,49 which assumes that the experimental stimulus has the same effect on the BOLD signal for every subject; the other one is a random-effects model,50 which assumes that the experimental stimulus could have a different effect on each subject, and the effect across subjects fits to a specific distribution, typically a normal distribution. Our experimental results do not fit the fixed-effects analysis. The intersubject variability in the patient group may also be due to a combination of factors such age, lesion size, and acuity, as well as differences in cognitive strategies.51 Even among control subjects who achieve very similar performance levels in each paradigm, and are of similar age and acuities (as in the case of control subjects 101 and 102), the activation patterns analyzed across the lobes of the brain show differences that suggest different networking strategies for processing visual tasks.
Control for this intersubject variability is required to perform group analyses. A recent study conducted by Raemaekers and colleagues52 found good intersubject reproducibility after individually addressing the global temporal signal-to-noise ratio (SNR) for each subject. The global temporal SNR is represented by the average t value across all activated voxels in the whole brain. They propose the intersubject variability can largely be explained by the intersubject variability in temporal SNR. Although the brain activation variability may also be due to the different cognitive strategies, their method provides a possible way to create a general brain activation pattern in patients with macular degeneration. Our approach of using regional activation proportions of the total brain activation is a simple normalization method that can be applied when SNR is reproducible with stable scanner performance and subjects who are well trained with the paradigms and imaging environment. Brain activation clustering algorithms,53–55 which can separate the subjects into subgroups with more homogeneous brain activation patterns, provides another way to perform more accurate group analysis.
In conclusion, the significant repeatability of the fMRI brain activation patterns from our paradigms within individuals shows that fMRI can possibly serve as a reliable measure of cortical function and has potential for measuring outcomes of vision rehabilitation. Furthermore, this study has suggested that the activation proportion method could serve as a reliable index to measure reproducibility. The poorer intrasubject reproducibility of patients with macular degeneration in visually guided saccade tasks suggests that these patients may lack a successful oculomotor processing strategy for coordinating eye movement for finding and detecting a target. The implication of these findings is that the success of training visually compromised patients to use specific eccentric viewing strategies to maximize use of their PRL may be reflected by improved performance and a reduction in the variability of brain activation patterns. Considering the striking intersubject variability, even in control subjects, individual analysis is necessary when conducting longitudinal brain activation studies for the patients using fMRI, and group mapping should be applied only after considering the intersubject variability.
Acknowledgments
The authors thank Marlos Viana, PhD, for his advice on the methods of statistical analysis and Gerald A. Fishman, MD, for his referral of the patients with Stargardt disease.
Footnotes
Supported in part by grants from the Department of Veterans Affairs, Rehabilitation Research and Development Service (Washington, DC); Washington Square Health Foundation (Chicago, Illinois); The Cless Family Foundation (Northbrook, Illinois); Research to Prevent Blindness, Inc. (New York, New York); and National Eye Institute Core Grant EY01792.
Disclosure: J. Ming, None; K.R. Thulborn, None; J.P. Szlyk, None
References
- 1.Bressler N, Bressler S, Fine S. Age-related macular degeneration. Surv Ophthalmol. 1988;32:375–413 [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein B, Tomany S, et al. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2002;109:1767–1779 [DOI] [PubMed] [Google Scholar]
- 3.Stelmack J, Szlyk J, Stelmack T. Psychometric properties of the veterans affairs low-vision visual functioning questionnaire (VA LV VFQ-48). Invest Ophthalmol Vis Sci. 2004;45:3919–3928 [DOI] [PubMed] [Google Scholar]
- 4.Szlyk J, Stelmack J, Massof R. Performance of the Veterans Affairs Low Vision Visual Functioning Questionnaire (VA LV VFQ). J Vis Impairment Blindness. 2004;98:261–275 [Google Scholar]
- 5.Crossland M, Culham L, Kabanarou S, Rubin G. Preferred retinal locus development in patients with macular disease. Ophthalmology. 2005;112:1579–1585 [DOI] [PubMed] [Google Scholar]
- 6.Fletcher D, Schuchard R, Watson G. Relative locations of macular scotomas near the PRL: effect on low vision reading. J Rehab Res Dev. 1999;36:356–364 [PubMed] [Google Scholar]
- 7.Little D, Thulborn K, Szlyk J. An fMRI study of saccadic and smooth pursuit eye movement control in patients with AMD. Invest Ophthalmol Vis Sci. 2008;49:1728–1735 [DOI] [PubMed] [Google Scholar]
- 8.Szlyk JP, Seiple W, Viana M. Relative effects of age and compromised vision on driving performance. Hum Factors. 1995;37:430–436 [DOI] [PubMed] [Google Scholar]
- 9.Szlyk JP, Fishman GA, Grover S, Revelins BI, Derlacki DJ. Difficulty in performing everyday activities in patients with juvenile macular dystrophies: comparison with patients with retinitis pigmentosa. Br J Ophthalmol. 1998;82:1372–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casco C, Campana G, Grieco A, Musetti S, Perrone S. Hyper-vision in a patient with central and paracentral vision loss reflects cortical reorganization. Vis Neurosci. 2003;20:501–510 [DOI] [PubMed] [Google Scholar]
- 11.Thulborn KR, Davis D, Erb P, Strojwas M, Sweeney JA. Clinical fMRI: implementation and experience. Neuroimage. 1996;4:S101–S107 [DOI] [PubMed] [Google Scholar]
- 12.Detre JA, Floyd TF. Functional MRI and its applications to the clinical neurosciences. Neuroscientist. 2001;7:64–79 [DOI] [PubMed] [Google Scholar]
- 13.Powell HW, Duncan JS. Functional magnetic resonance imaging for assessment of language and memory in clinical practice. Curr Opin Neurol. 2005;18:161–166 [DOI] [PubMed] [Google Scholar]
- 14.Price CJ, Crinion J. The latest on functional imaging studies of aphasic stroke. Curr Opin Neurol. 2005;18:429–434 [DOI] [PubMed] [Google Scholar]
- 15.Mitterschiffthaler MT, Ettinger U, Mehta MA, Mataix-Cols D, Williams SC. Applications of functional magnetic resonance imaging in psychiatry. J Magn Reson Imaging. 2006;23:851–856 [DOI] [PubMed] [Google Scholar]
- 16.Cabeza R, Nyberg L Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47 [DOI] [PubMed] [Google Scholar]
- 17.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwong KK, Belliveau JW, Chesler DA. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–397 [DOI] [PubMed] [Google Scholar]
- 20.Sunness JS, Liu T, Yantis S. Retinotopic mapping of the visual cortex using functional magnetic resonance imaging in a patient with central scotomas from atrophic macular degeneration. Ophthalmology. 2004;111:1595–1598 [DOI] [PubMed] [Google Scholar]
- 21.Baker CI, Peli E, Knouf N, Kanwisher NG. Reorganization of visual processing in macular degeneration. J Neurosci. 2005;25:614–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung SH, Schuchard RA, He S, Tai Y, Legge GE, Hu XP. Limited retinotopic reorganization in age-related macular degeneration. J Vis. 2005;5:299 [Google Scholar]
- 23.Masuda Y, Nakadomari S, Asakawa K, et al. Lack of cortical reorganization in macular degeneration patients. 2006. Poster session presented at Society for Neuroscience, Atlanta, GA. [Google Scholar]
- 24.Baker CI, Dilks DD, Peli E, Kanwisher N. Reorganization of visual processing in macular degeneration: replication and clues about the role of foveal loss. Vision Res. 2008;48:1910–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuda Y, Dumoulin SO, Nakadomari S, Wandell BA. V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cereb Cortex. 2008;18:2483–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dilks DD, Baker CI, Peli E, Kanwisher N. Reorganization of visual processing in macular degeneration is not specific to the “preferred retinal locus.” J Neurosci. 2009;29:2768–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little D, Thulborn K, Szlyk J. An fMRI study of saccadic and smooth pursuit eye movement control in patients with AMD. Invest Ophthalmol Vis Sci. 2008;49:1728–1735 [DOI] [PubMed] [Google Scholar]
- 28.Szlyk JP, Little DM. An fMRI study of word-level recognition and processing in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:4487–4495 [DOI] [PubMed] [Google Scholar]
- 29.Seiple W, Szlyk JP, McMahon T, Pulido J, Fishman GA. Eye-movement training for reading in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005;46:2886–2896 [DOI] [PubMed] [Google Scholar]
- 30.Brannen JH, Badie B, Moritz CH, Quigley M, Meyerand ME, Haughton VM. Reliability of functional MR imaging with word-generation tasks for mapping broca's area. Am J Neuroradiol. 2001;22:1711–1718 [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez G, Specht K, Weis S, et al. Intrasubject reproducibility of presurgical language lateralization and mapping using fMRI. Neurology. 2003;60:969–975 [DOI] [PubMed] [Google Scholar]
- 32.Rutten GJ, Ramsey NF, van Rijen PC, van Veelen CW. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain Lang. 2002;80:421–437 [DOI] [PubMed] [Google Scholar]
- 33.Specht K, Willmes K, Shah NJ, Jancke L. Assessment of reliability in functional imaging studies. J Magn Reson Imaging. 2003;17:463–471 [DOI] [PubMed] [Google Scholar]
- 34.Cohen MS, DuBois RM. Stability, repeatability, and the expression of signal magnitude in functional magnetic resonance imaging. J Magn Reson Imaging. 1999;10:33–40 [DOI] [PubMed] [Google Scholar]
- 35.Ramsey NF, Tallent K, VanGelderen P, Frank JA, Moonen CT, Weinberger DR. Reproducibility of 3D fMRI brain maps acquired during a motor task. Hum Brain Mapp. 1996;4:113–121 [DOI] [PubMed] [Google Scholar]
- 36.Tegeler C, Strother SC, Anderson JR, Kim SG. Reproducibility of BOLD-based functional MRI obtained at 4 T. Hum Brain Mapp. 1999;7:267–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baseler HA, Gouws A, Morland AB. The organization of the visual cortex in patients with scotomata resulting from lesions of the central retina. J Neuroophthalmol. 2009;33:149–157 [Google Scholar]
- 38.Baseler HA, Gouws A, Haak KV, et al. Large-scale remapping of visual cortex is absent in adult humans with macular degeneration. Nat Neurosci. 2011a;14:649–655 [DOI] [PubMed] [Google Scholar]
- 39.Baseler HA, Gouws A, Crossland MD, et al. Objective visual assessment of antiangiogenic treatment for wet age-related macular degeneration. Optom Vis Sci. 2011b;88:1255–1261 [DOI] [PubMed] [Google Scholar]
- 40.Rebecca LB, Panagiotis GS, Andrew CP. Reliability and validity of functional neuroimaging techniques for identifying language-critical areas in children and adults. Dev Neuropsychol. 2004;26:541–556 [DOI] [PubMed] [Google Scholar]
- 41.Toft P. Quantitative MRI of the Brain: Measuring Changes Caused by Disease. Chichester, UK: John Wiley & Sons Ltd; 2003:442–443 [Google Scholar]
- 42.Chen EE, Small SL. Test-retest reliability in fMRI of language: group and task effects. Brain Lang. 2007;102:176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manoach DS, Halpern EF, Kramer TS, et al. Test-retest reliability of a functional MRI working memory paradigm in normal and schizophrenic subjects. Am J Psychiatry. 2001;158:955–958 [DOI] [PubMed] [Google Scholar]
- 44.Kimberley TJ, Khandekar G, Borich M. fMRI reliability in subjects with stroke. Exp Brain Res. 2008;186:183–190 [DOI] [PubMed] [Google Scholar]
- 45.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173 [DOI] [PubMed] [Google Scholar]
- 46.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428 [DOI] [PubMed] [Google Scholar]
- 47.Wei X, Yoo SS, Dickey CC, Zou KH, Guttmann CR, Panych LP. Functional MRI of auditory verbal working memory: long-term reproducibility analysis. Neuroimage. 2004;21:1000–1008 [DOI] [PubMed] [Google Scholar]
- 48.Miki A, Raz J, van Erp TG, Liu CS, Haselgrove JC, Liu GT. Reproducibility of visual activation in functional MR imaging and effects of postprocessing. Am J Neuroradiol. 2000;21:910–915 [PMC free article] [PubMed] [Google Scholar]
- 49.Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396 [DOI] [PubMed] [Google Scholar]
- 50.Holmes AP, Friston KJ. Generalisability, random effects, and population inference. Neuroimage. 1998;7:S754 [Google Scholar]
- 51.Crossland MD, Morland AB, Feely MP, von dem Hagen E, Rubin GS. The effect of age and fixation instability on retinotopic mapping of primary visual cortex. Invest Ophthalmol Vis Sci. 2008;49:3734–3739 [DOI] [PubMed] [Google Scholar]
- 52.Raemaekers M, Vink M, Zandbelt B, van Wezel RJ, Kahn RS, Ramsey NF. Test-retest reliability of fMRI activation during prosaccades and antisaccades. Neuroimage. 2007;36:532–542 [DOI] [PubMed] [Google Scholar]
- 53.Noppeney U, Penny WD, Price CJ, Flandin G, Friston KJ. Identification of degenerate neuronal systems based on intersubject variability. Neuroimage. 2006;30:885–890 [DOI] [PubMed] [Google Scholar]
- 54.Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Structural group classification technique based on regional fMRI BOLD responses. IEEE Trans Med Imaging. 2005;24:389–398 [DOI] [PubMed] [Google Scholar]
- 55.Zhong N, Wu JL, Nakamura A, Ohshima M, Mizuhara H. Peculiarity oriented fMRI brain data analysis for studying human multi-perception mechanism. Cogn Syst Res. 2004;5:241–256 [Google Scholar]