Abstract
The trigeminal autonomic cephalalgias (TACs) are a group of primary headache disorders that are characterized by strictly unilateral trigeminal distribution pain occurring in association with ipsilateral cranial autonomic symptoms. This group includes cluster headache, paroxysmal hemicrania and short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing. These disorders are very painful, often considered to be some of the most painful conditions known to mankind, and consequently are highly disabling. They are distinguished by the frequency of attacks of pain, the length of the attacks and very characteristic responses to medical therapy, such that the diagnosis can usually be made clinically, which is important because it dictates therapy. The management of TACs can be very rewarding for physicians and highly beneficial to patients.
Keywords: Cluster headache, paroxysmal hemicrania, SUNA, SUNCT, trigeminal autonomic cephalalgias
Introduction
The trigeminal autonomic cephalalgias (TACs) are a group of primary headache disorders characterized by unilateral head pain that occurs in association with generally prominent ipsilateral cranial autonomic features.[1] The TACs include cluster headache (CH), paroxysmal hemicrania (PH), short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) and its close relative, short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA). CH, PH and SUNCT are currently grouped into section 3 of the revised International Classification of Headache Disorders (ICHD-II), while SUNA is described in the appendix section. While SUNCT and SUNA are currently considered to be TACs, it has been argued that these disorders are trigeminal neuralgia variants and, therefore, may be more appropriately grouped among the cranial neuralgias.[2]
CH, PH and SUNCT are characterized by short-lasting headaches with autonomic features. Despite their common elements, these three TACs differ in attack duration and frequency as well as response to therapy. CH has the longest attack duration and relatively low attack frequency. PH has intermediate duration and intermediate attack frequency. SUNCT has the shortest attack duration and the highest attack frequency. The importance of recognizing these syndromes resides in their excellent, but highly selective, response to treatment.
Cluster Headache
CH is a strictly unilateral headache that occurs in association with cranial autonomic features and, in most patients, has a striking circannual and circadian periodicity. It is an excruciating syndrome and is probably one of the most painful conditions known to mankind, with female patients describing each attack as being worse than childbirth.
Epidemiology
The prevalence of CH is estimated to be 0.1%,[3] although a recent study suggests that the prevalence of CH may be as high as two per 1000.[4] The male:female ratio is 2.5:1.[5] It can begin at any age, though the most common age of onset is the third or fourth decade of life.
Clinical features
Cluster attacks are strictly unilateral, although the headache may alternate sides. The pain is excruciatingly severe. It is located mainly around the orbital and temporal regions, although any part of the head can be affected. The headache usually lasts 45–90 min, but can range from 15 min to 3 h. It has an abrupt onset and cessation, and attacks are accompanied by cranial autonomic symptoms. Migrainous symptoms, such as nausea, vomiting, photophobia and phonophobia, are seen in significant proportions of cluster patients,[5,6] and aura has also been reported.[7] The vast majority of CH patients report restlessness or even aggressiveness during the attacks[5] and, therefore, this feature has been incorporated into the ICHD-II diagnostic criteria.[8] The condition can have a striking circadian rhythmicity, with some patients reporting that the attacks occur at the same time each day.
Alcohol, nitroglycerine, exercise and elevated environmental temperature are recognized precipitants of acute cluster attacks. Alcohol induces acute attacks, usually within 1 h of intake, in the vast majority of sufferers, contrasting with migraine sufferers who generally have headache some hours after alcohol intake. Alcohol triggers attacks during a cluster bout, but not in a remission.
Classification
CH is classified according to the duration of the bout. About 80–90% of the patients have episodic cluster headache (ECH), which is diagnosed when they experience recurrent bouts, each with a duration of more than a week and separated by remissions lasting more than 4 weeks. The bouts typically occur once or twice a year. The remaining 10–20% of the patients have chronic cluster headache (CCH), in which either no remission occurs within 1 year or the remissions last less than 1 month.[8]
Differential diagnosis
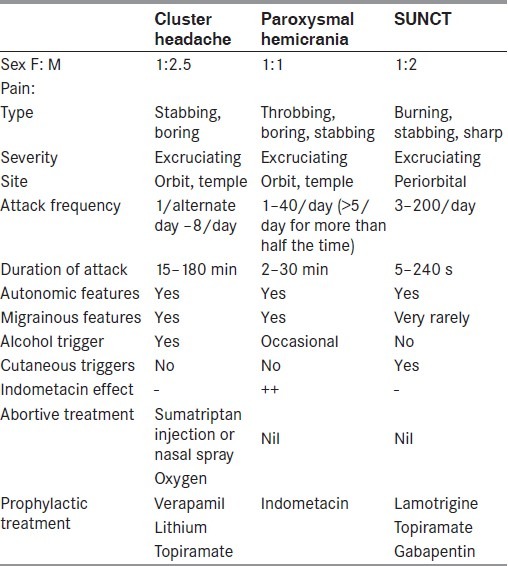
The major differential diagnostic considerations are the other TACs [Table 1] and secondary causes of CH. The vast majority of CH patients have a primary headache syndrome, with symptomatic causes only being identified in a very small minority. However, the true prevalence of symptomatic causes of CH is unknown as there are no prospective population-based neuroimaging studies. A review of retrospective case reports published in the medical literature suggests that the TACs may be associated with pituitary tumors, although this most likely reflects a considerable element of publication bias.[9] Similarly, an observational study of headache disorders in patients with pituitary tumors reported that CH occurred in 4% and SUNCT in 5%, but the study was conducted in a tertiary referral neurosurgical center and, therefore, does not give a meaningful indication of the prevalence of these headaches in patients with pituitary disorders.[10] It remains unclear whether every TAC patient requires neuroimaging, although, if it is considered, then magnetic resonance imaging (MRI) is the preferred modality. Some authors suggest that all patients with TACs should have dedicated pituitary imaging. However, approximately one in 10 of the general population has an incidental pituitary microadenoma (<1 cm diameter) on routine MRI, and up to one in 500 will have a macroadenoma.[11] This approach is therefore likely to identify a significant number of incidental lesions, which could then be erroneously considered to be the cause of the TAC syndrome. We suggest that all TAC patients should be carefully assessed for pituitary disease-related symptoms and that further investigations with MRI of the pituitary gland should be undertaken in patients with atypical features, abnormal examination or those resistant to the appropriate medical treatments.
Table 1.
Clinical features of the trigeminal autonomic cephalalgias
Treatment
Abortive agents
Subcutaneous sumatriptan
Subcutaneous sumatriptan 6 mg is the drug of choice as abortive treatment of a cluster attack.[12] In CH, unlike in migraine, subcutaneous sumatriptan can be prescribed at a frequency of twice daily, on a long-term basis if necessary, without risk of tachyphylaxis or rebound.[13,14]
Oxygen
0Inhalation of 100% oxygen, at 7–12 L/min, is rapidly effective in relieving pain in the majority of sufferers.[15–17] It should be inhaled continuously for 15–30 min via a nonrebreathing facial mask. However, up to 25% of the patients note that oxygen simply delays the attack for minutes to hours rather than completely aborting it.[15]
Intranasal triptans
Sumatriptan nasal spray (20 mg) and zolmitriptan nasal spray (5 mg and 10 mg) are both more effective than placebo.[18–20] Given the efficacy of both zolmitriptan 5 mg and 10 mg doses, it has been advised that 10 mg might be the optimal initial dose for those with very severe attacks occurring only once per day or every other day, while 5 mg should be the initial dose for those with more frequent attacks or poor tolerability.
Topical lidocaine
Lidocaine solution, given as nasal drops (10% lidocaine solution) or a spray deep in the nostril on the painful side, has been reported to give mild to moderate relief in patients during a CH attack, although only a few patients obtain complete pain relief.[21–22] Therefore, intranasal lidocaine serves as a useful adjunct to other abortive treatments, but is rarely adequate on its own.
Dihydroergotamine nasal spray
Dihydroergotamine (DHE) nasal spray 1 mg has been studied in a double-blind, placebo-controlled, crossover trial.[23] There was no difference in the headache frequency or duration, but the pain intensity was significantly reduced with DHE compared with placebo. The dosage used (1 mg) was rather low; therefore, DHE nasal spray at a dose of 2 mg or 4 mg may be more effective than 1 mg, although this needs to be studied in a controlled fashion.
A novel inhaled formulation of DHE (MAP0004), which has a comparable time to peak concentration and area under the curve with intravenous DHE, has been shown to be effective in aborting migraine attacks in a recent phase 3 double-blind placebo-controlled trial.[24] Because of the restricted choice of acute treatments for CH attacks, MAP0004 should be considered in future CH trials.
Transitional treatments
There can be a lag of several days to a few weeks before the efficacy of preventive treatments becomes apparent. Transitional treatments, which produce a rapid suppression of the attacks for a limited period of time or cannot be used for prolonged periods, can be used when waiting for the beneficial effect of a preventive treatment to become evident. Transitional treatments can be also be used in patients with ECH to treat relatively short bouts (≤1 month), without the need to start a preventive drug.
Corticosteroids
Several investigators have reported the beneficial effect of oral or parenteral corticosteroid regimens in the treatment of CH.[25,26] The methodological quality of these studies is low, with uncontrolled and inconclusive studies being the norm. However, these studies have nearly uniformly reported positive treatment effects, and this is consistent with the clinical experiences of most physicians caring for CH patients.[27] Caution has to be exercised in their use because of the potential for serious side-effects. Thus, a tapering course of prednisone or prednisolone for 3 weeks is prudent. Unfortunately, relapse almost invariably occurs as the dose is tapered. For this reason, steroids are used as an initial therapy in conjunction with preventives, until the latter are effective. We start patients on oral prednisone 1 mg/kg, to a maximum of 60 mg a day, for 5 days and thereafter decrease the dose by 10 mg every 3 days.
Greater occipital nerve block
A double-blind, placebo-controlled study of suboccipital injection with a mixture of rapid- and long-acting betamethasone have been performed in CH.[28] The authors studied 16 ECH and 7 CCH patients. Eleven of 13 (85%) CH patients treated with betamethasone suboccipital injection became pain-free within 1 week compared with none of the 10 patients treated with placebo injection. This effect was maintained for at least 4 weeks in the majority of the patients. Given the relatively good evidence of efficacy, suboccipital steroid injection can be considered in the treatment of CH.[29]
Intravenous dihydroergotamine
Repetitive intravenous DHE administered to inpatients over a period of 3 days was reported to be very useful in some cases of both ECH and CCH. In a study of 54 patients with intractable CH (23 episodic, 31 chronic), the open-label use of repetitive intravenous DHE rendered all patients headache free.[30] At 12-month follow-up, 83% and 39% of the patients with ECH and CCH, respectively, remained free of headache. A retrospective analysis evaluated the efficacy and safety of intravenous DHE for the treatment of refractory CH in 70 patients,[31] and showed a complete resolution of the pain at 1 month after treatment in 62% of the cases, partial improvement in 14% and failure in 24%. Side-effects were transient and well tolerated in most patients.
Preventive treatments
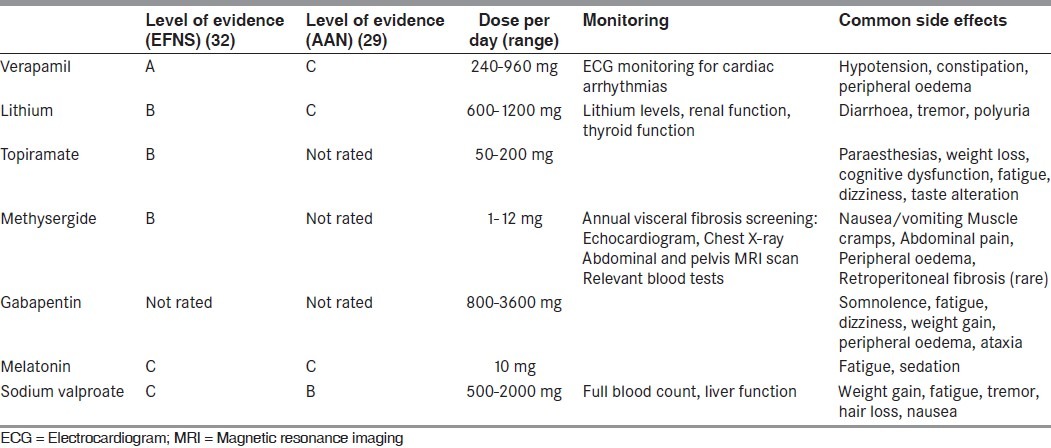
The preventive agents used include verapamil, lithium, topiramate, methysergide, gabapentin, melatonin and valproate. Verapamil is the first-line agent of choice. Second-line agents include lithium and topiramate, while methysergide is a reasonable choice for a third-line agent. Table 2 provides an overview of the recommendations for CH preventive treatments by the European Federation of Neurological Societies (EFNS)[32] and the American Academy of Neurology (AAN).[29]
Table 2.
Preventive treatments of cluster headache
Verapamil
Verapamil is the preventative drug of choice in both episodic and chronic CH.[33,34] Dosages commonly employed range from 240 mg to 960 mg in divided doses (two or three times a day). Verapamil can cause heart block by slowing conduction in the atrioventricular node. Observing for PR interval prolongation on ECG can monitor potential development of heart block. There is only one formal guideline in the literature for the titration of the verapamil dose.[35] After performing a baseline ECG, patients are usually started on 80 mg tds and, thereafter, the total daily dose is increased in increments of 80 mg every 10-14 days. An ECG is performed prior to each increment. The dose is increased until the cluster attacks are suppressed, side-effects intervene or the maximum dose of 960 mg daily is achieved. ECG monitoring should be performed periodically in patients on long-term verapamil.
Lithium
Lithium is an effective agent for CH prophylaxis, although the response is less-robust in ECH than in CCH.[33,36] Most patients will benefit from dosages between 600 mg and 1200 mg daily or at plasma concentrations comprised between 0.8 mEq/L and 1.0 mEq/L. Renal and thyroid function tests are performed prior and during treatment in view of the long-term risk of hypothyroidism and nephrogenic diabetes insipidus.
Topiramate
Five open-label studies have reported the efficacy of topiramate in the preventive treatment of CH.[37–41] The dose of topiramate used in these studies ranged from 25 mg to 250 mg daily. The side-effect profile of this agent, including cognitive slowing and depression, often limits its use.
Methysergide
Methysergide has long been used for the treatment of CH.[25,42] It is an ideal choice in patients with short cluster bouts, which last less than 4–5 months. Doses up to 12 mg daily can be used, if tolerated. Prolonged treatment has been associated with fibrotic reactions (retroperitoneal, pulmonary, pleural and cardiac), although these are rare.[22] We advise a 1-month holiday every 6 months of therapy and check for evidence of pulmonary, cardiac, renal or abdominal pathology yearly if repetitive courses of treatment are required over a prolonged period.
Other preventive treatments
In a double-blind, placebo-controlled trial of melatonin 10 mg, five of 10 subjects randomized to melatonin were rendered pain free within 5 days, while none of the 10 subjects taking placebo derived any benefit.[43] Recently, Peres and Rozen[44] reported two CCH patients inadequately managed on verapamil 640 mg daily who were rendered pain free with add-on therapy with melatonin 9 mg daily. The authors concluded that melatonin could be a useful adjunctive treatment for CH prophylaxis.
Gabapentin was tried at the dose of 900 mg/day in an open-label fashion in eight ECH and four CCH patients.[45] All patients were rendered pain free within 8 days of initiating therapy. Patients with ECH discontinued gabapentin after 60 days of treatment without recurrence of the attacks. The four CCH patients remained pain free at follow-up of 4 months. This astonishingly high response rate needs to be reproduced in controlled trials.
Surgery
Surgical options are the measures of last resort in medically intractable patients, and should only be considered when the pharmacological options have been exploited to the fullest.[49] Historically, destructive procedures, like trigeminal sensory rhizotomy and radiofrequency trigeminal ganglio-rhizolysis, have been tried in CH.[50,51] However, they are associated with considerable morbidity and therefore have been largely abandoned. Neurostimulation therapies that entail peripheral or central nervous system targets are emerging as very promising approaches.
Occipital nerve stimulation
Studies conducted in small cohorts of medically intractable CCH patients have shown occipital nerve stimulation (ONS) to be a promising therapy.[52,53] Magis and colleagues[52] treated eight patients with medically intractable CCH using unilateral ONS. After a mean follow-up of 15 months, two patients were pain free, three patients had a 90% reduction in attack frequency while two patients had improvement of around 40%. Interruption of ONS was followed within days by recurrence and increase of attacks in all improved patients. Burns and colleagues[53] treated 14 patients with medically intractable CCH using bilateral ONS. At a median follow-up of 17.5 months, 10 of the 14 patients reported improvement that was sufficiently meaningful for them. Subjective self-reporting of improvement was 90% or more in three patients, 40–60% in three patients and 20–30% in four patients. Benefit from stimulation was not immediate, with maximal effect noted after several months.
Hypothalamic region deep brain stimulation
Based on the finding of ipsilateral posterior inferior hypothalamic activation in CH, various centers have treated intractable chronic CH patients by electrode implantation and stimulation of this region.[54–56] Leone and colleagues[57] have recently reviewed the results of hypothalamic region deep brain stimulation (DBS) in 38 patients; 23 patients (61%) were rendered pain free or almost pain free. In most patients, the headaches recurred when the stimulation was stopped. A French multicenter, randomized, double-blind, crossover study enrolled 12 patients, with only 11 undergoing surgery. Results were not encouraging in the blinded cross-over phase.[58] However, the crossover assignment was very short (only 1 month) and, in the open phase, six of 11 patients were considered responders.
CCH is a devastating illness and, given the low morbidity of ONS and the relatively consistent outcomes, one might argue that this modality should be explored before DBS, which is associated with a small risk of morbidity and mortality.
Paroxysmal Hemicrania
Paroxysmal hemicrania (PH), like CH, is characterized by strictly unilateral, brief, excruciating headaches that occur in association with cranial autonomic features. PH differs from CH mainly in the higher frequency and shorter duration of individual attacks, although there is a considerable overlap in these characteristics. However, unlike CH, PH responds in a dramatic and absolute fashion to indomethacin,[8] thereby underlining the importance of distinguishing it from CH.
Epidemiology
PH is a rare syndrome. However, with increasing awareness, it is being recognized more frequently. The prevalence of PH is not known, and seems to occur equally in females and males.[59] It can begin at any age, although the most common age of onset is the second or third decade of life.[60]
Clinical features
The attack profile of PH is highly characteristic.[59,61] The headache is strictly unilateral. The maximum pain is most often centered on the ocular, temporal, maxillary or frontal regions; less often, is the pain centered on the neck, occiput or the retro-orbital regions. The pain is typically excruciating in severity and described as a throbbing, aching or boring sensation. The headache usually lasts 10–30 min, but can range from 2 min to 45 min. It has an abrupt onset and cessation. Interictal discomfort or pain is present in up to 60% of the patients.[59]
Attacks of PH invariably occur in association with ipsilateral cranial autonomic features. The IHS classification criteria for chronic paroxysmal hemicrania require the attacks to be accompanied by at least one of the following, which have to be present on the pain side: Conjunctival injection, lacrimation, nasal congestion, rhinorrhoea, ptosis or eyelid edema.[8] Photophobia and nausea may accompany some attacks, although vomiting and phonophobia are rare. During episodes of pain, approximately 50–80% of the sufferers are agitated and restless, and one-quarter are described as being aggressive during the pain.[59,61]
In PH, the attacks occur at a high frequency. Typically, patients have more than five attacks daily, although the frequency of attacks shows a considerable fluctuation, ranging between one and 40 daily. The attacks occur regularly throughout the 24-h period, without a preponderance of nocturnal attacks as in CH.
While the majority of attacks are spontaneous, approximately 10% of the attacks may be precipitated mechanically, either by bending or by rotating the head. Attacks may also be provoked by external pressure against the transverse processes of C4-5, C2 root or the greater occipital nerve. Alcohol ingestion triggers headaches in only 7% of the patients.[61]
Classification
PH is classified depending on the presence of a remission period. About 20% of the patients have episodic paroxysmal hemicrania (EPH), which is diagnosed when there are clear remission periods between bouts of attacks. The remaining 80% of the patients have chronic paroxysmal hemicrania (CPH),[61] which is diagnosed when patients have either no remission within 1 year or the remissions last less than 1 month.
Differential diagnosis
The differential diagnoses that need to be considered are: Secondary causes of PH, other TACs and hemicrania continua (HC). PH can be differentiated from CH and SUNCT, with a trial of indometacin. HC is a strictly unilateral headache that is continuous and associated with ipsilateral cranial autonomic symptoms. Both PH and HC are exquisitely responsive to indometacin, and have to be differentiated on the basis of the clinical phenotype.[62]
A large number of symptomatic cases of PH have been described, although a causal relationship is difficult to ascertain in most of these cases.[62] An MRI brain scan is a reasonable screening test in all patients with PH. As with CH, an association with pituitary tumors has been reported. We suggest that all TAC patients should be carefully assessed for pituitary disease-related symptoms, but further investigations with MRI of the pituitary gland should only be undertaken in patients with atypical features, abnormal examination or those resistant to the appropriate medical treatments.
Treatment
Indometacin
The treatment of PH is prophylactic. Indometacin is the treatment of choice and, in fact, has been deemed the sine qua non for establishing the diagnosis.[8] Complete resolution of the headache is prompt, usually occurring within 1–2 days of initiating the effective dose. The typical maintenance dose ranges from 25 mg to 100 mg per day, but doses up to 300 mg daily are occasionally required.[59] In patients with EPH, indometacin should be given for slightly longer than the typical headache bout and then gradually tapered. In patients with CPH, long-term treatment is usually necessary, although drug withdrawal should be advised at least once every 6 months. Gastroprotective agents should always be considered for patients who require long-term treatment.
To circumvent some of the problems with oral indometacin administration (i.e., difficulties with achieving adequate dose due to side-effects), an intramuscular trial of indometacin, the “indotest,” has been shown to be a rapid and useful test for PH and HC,[63] although the role of a placebo response is not defined. Therefore, a modified indotest (placebo-controlled intramuscular indometacin 100 mg) has been proposed and validated for HC.[64] The modified indotest has been shown to be a useful alternative to oral indometacin also in a series of PH patients.[59]
Other medications
There has been some limited success in the treatment of PH with cyclooxygenase-2 (COX-2) inhibitors, rofecoxib[65–67] and celecoxib.[67,68] However, prolonged use of both of these agents has recently been linked with an increased risk of myocardial infarctions and strokes, and this culminated in the withdrawal of rofecoxib from the market worldwide.[69] In view of this, the available COX-2 inhibitors should be prescribed only with great caution in PH.
Topiramate has been found to be effective in two cases of PH,[70] and its efficacy also reflects our personal clinical experience.
Greater occipital nerve block has been described as helpful in this condition[71,72] and can, therefore, be tried, especially in view of its relatively safe adverse effect profile. Further data are necessary in order to clarify the consistency of its effect in PH.
SUNCT and SUNA
SUNCT is a rare primary headache syndrome, in which the pain has to be associated, by definition, with both ipsilateral conjunctival injection and lacrimation.[8] In recognition of the possibility that all patients with generically the same condition might not have both conjunctival injection and tearing, the classification committee considered that SUNCT syndrome may be a subset of SUNA. In SUNA, there may be cranial autonomic symptoms other than conjunctival injection and lacrimation, or indeed only one of those symptoms may be present. However, SUNA needs to be properly validated yet.
Epidemiology
SUNCT is relatively rare, with a recent study showing a prevalence of 6.6/100,000 and an incidence of 1.2/100,000.[73] The disorder has a male preponderance, with a sex ratio of 2:1.[74] The typical age of onset is between 40 and 70 years, with a mean age of onset at 48 years.
Clinical features
The pain is usually maximal in the ophthalmic distribution of the trigeminal nerve, especially the orbital or periorbital regions, forehead and temple. The attacks are strictly unilateral. The severity of pain is generally severe to excruciating. The pain is usually described as stabbing, burning, pricking or electric shock-like in character. The individual attacks are very brief, lasting for 5–240 s, and have one of three different types of attack profiles: They can occur as single short-lasting stabs; longer-lasting groups of repetitive stabs; or a serrated pattern.[74] Most patients are completely pain-free between attacks, although in a large series of SUNCT patients, 46% reported a persistent dull interictal discomfort.[74]
The temporal pattern is quite variable, with the symptomatic periods alternating with remissions in an erratic manner. Symptomatic periods generally last from a few days to several months, and occur once or twice annually. Remissions typically last a few months, although they can range from 1 week to 7 years. Symptomatic periods appear to increase in frequency and duration over time.[75]
The attack frequency during the symptomatic phase varies immensely between sufferers and within an individual sufferer. Attacks may be as infrequent as once a day or less to more than 30 attacks an hour. Most SUNCT attacks occur during the daytime; however, 40% of the patients reported nocturnal attacks as well.[74]
Acute headache episodes in SUNCT syndrome are accompanied by a variety of associated symptoms. The attacks are virtually always accompanied by both ipsilateral conjunctival injection and lacrimation. Ipsilateral nasal congestion, rhinorrhoea, eyelid edema, ptosis and facial redness or sweating are less commonly reported. These cranial autonomic symptoms, particularly conjunctival injection and lacrimation, are typically very prominent in SUNCT syndrome.[76] A combination of nausea, vomiting, photophobia and phonophobia are reported in 9% of SUNCT patients.[74] Restlessness was not considered a feature of SUNCT syndrome,[76] although a recent clinical study reported agitation during the attacks in 62% of the SUNCT patients.[74]
The majority of patients can precipitate attacks by touching certain trigger zones within trigeminal innervated distribution and, occasionally, even from an extratrigeminal territory.[75] Precipitants include touching the face or scalp, washing, shaving, eating, chewing, brushing teeth, talking and coughing.[76] Neck movements can also precipitate attacks, although some patients can lessen or abort attacks by continuously rotating their neck.[76] Unlike in trigeminal neuralgia, most patients have no refractory period.[74]
Differential diagnosis
The differential diagnosis of very brief headaches includes SUNCT (primary and secondary forms), trigeminal neuralgia, primary stabbing headache and PH.
Secondary SUNCT is typically seen with either posterior fossa[75] or pituitary gland lesions.[74,77,78] An observational study that defined the headache characteristics in pituitary tumor patients reported SUNCT-like phenotype in 5% of these patients, although the patient population studied was not representative of pituitary tumor patients as the study was performed in a tertiary referral neurosurgical setting.[10] Interestingly, Williams and Broadley, who systematically looked for trigeminal neurovascular conflict with dedicated trigeminal MRI scans, found a high proportion of ipsilateral vascular loops in contact with the trigeminal nerve in SUNCT and SUNA.[73] Therefore, a full diagnostic work-up for SUNCT/SUNA must include a brain MRI scan with dedicated trigeminal views and a trial of indometacin to exclude indometacin-responsive headaches. As with CH and PH, all SUNCT/SUNA patients should be carefully assessed for pituitary disease-related symptoms, but further investigations with MRI of the pituitary gland should only be undertaken in patients with atypical features, abnormal examination or those resistant to the appropriate medical treatments.
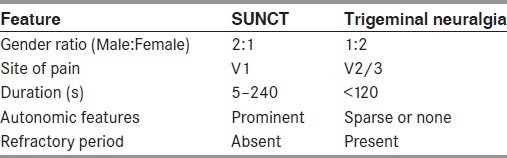
Differentiating SUNCT from trigeminal neuralgia can be difficult, as there is a considerable overlap in the clinical phenotypes of the two syndromes; indeed, some authors consider SUNCT to be a trigeminal neuralgia variant.[2] While the nosological status of SUNCT and SUNA remains unclear, there are some clinical features that can aid in the differentiation of these disorders. These features are outlined in Table 3.
Table 3.
Differentiating features of short-lasting neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) and trigeminal neuralgia
Treatment
Transitional treatments
Intravenous lidocaine
Intravenous lidocaine has been reported to quickly and completely abort attacks of SUNCT and SUNA.[73,79,80] The mean duration of the longest pain-free period after the end of the infusion has been reported to be 3 weeks in a patient with chronic SUNCT, 12 weeks pain free in chronic SUNA and 6 months pain free in a patient with episodic SUNCT.[81] We advice the use of lidocaine as a short-term treatment in patients who present in a so-called “SUNCT status”[82] and also in order to avoid breakthrough attacks while switching from a preventive drug to another in patients with high load of attacks. The dose recommended varies from 1.3 mg/kg/h to 3.3 mg/kg/h for 7–10 days, and the usual effective infusion speed varies from 15 ml/h to 30 ml/h.
Greater occipital nerve blocks
A suboccipital injection of a combination of lidocaine and a steroid was beneficial in five of eight SUNCT patients.[80] Greater occipital nerve (GON) injections may render the patient pain free for weeks or months, which would allow for the introduction and dose escalation of preventive medications.
Preventive treatments
There are no published placebo-controlled trials of preventive treatments in SUNCT/SUNA. In view of the rarity of this condition, most of the findings are based on cases reports or very small case series.
Lamotrigine
Lamotrigine is the treatment of choice for SUNCT and SUNA.[73,80,83] Problems with lamotrigine include a skin reaction that may progress to Stevens–Johnson syndrome, and this necessitated the cessation of the drug in at least one patient in the literature.[84]
Topiramate
Topiramate has been reported to be effective at doses up to 400 mg daily in 11 of 21 SUNCT patients in an open-label study.[80]
Gabapentin
SUNCT has been shown to respond to gabapentin, with complete suppression of attacks in three of nine patients treated with 800–2700 mg daily;[85–87] 10 of 22 SUNCT patients have also reported an improvement with this drug in an open-label trial.[80]
Surgery
Several surgical approaches have been tried in SUNCT syndrome. The approaches attempted can be subdivided into two main groups: Invasive procedures involving the trigeminal nerve and neuromodulation techniques.
Trigeminal procedures
Procedures that have been reported to be effective in SUNCT syndrome include: Percutaneous trigeminal ganglion compression,[88] trigeminal ganglion thermocoagulation,[89] retrogasserian glycerol rhizolysis[90] and gamma knife surgery.[91] These procedures provided complete pain relief, although the duration of the benefit ranged from 3 months to 4.5 years. Conversely, there are four reports of patients who were submitted to trigeminal procedures without any benefit.[90,92]
There have been four case reports of successful microvascular decompression with follow-up periods ranging from 3 months to 2 years.[93–96] Moreover, in a recent study, nine medically intractable chronic SUNCT/SUNA patients, who had an aberrant loop in contact with the symptomatic trigeminal nerve, underwent a microvascular decompression. Six of nine cases became completely pain free immediately after the operation, and the efficacy was sustained for a follow-up of 9–32 months. Only minor complications followed the surgery.[97]
Occipital nerve stimulation
Matharu and colleagues[98] reported the outcome of seven medically intractable SUNCT and one SUNA patient treated with bilateral ONS. These patients failed to respond to several preventive treatments. At a median follow-up of 24 months (range 4–29), five patients reported a moderate to substantial improvement. No major adverse events were reported.
Hypothalamic region deep brain stimulation
Based on the finding of posterior hypothalamic region activation in SUNCT,[99] two medically intractable SUNCT patients have been treated with posterior hypothalamus DBS.[100,101] The patients were reported to have responded well and the procedure was well tolerated. However, more data are required before hypothalamic region DBS can be routinely recommended.
Pathophysiology of TACs
The trigemino–autonomic reflex and hypothalamic activation
Any pathophysiological construct for TACs must account for the three major clinical features characteristic of the various conditions that comprise this group: Trigeminal distribution pain; ipsilateral autonomic features; and, the distinct circadian and circannual periodicity, especially in CH. The pain-producing innervation of the cranium projects through branches of the trigeminal and upper cervical nerves to the trigeminocervical complex, from where the nociceptive pathways project to higher centers. This implies an integral role for the ipsilateral trigeminal nociceptive pathways in TACs. The ipsilateral autonomic features suggest cranial parasympathetic activation (lacrimation, rhinorrhoea, nasal congestion and eyelid edema) and sympathetic hypofunction (ptosis and miosis). Goadsby and Lipton have suggested that the pathophysiology of the TACs revolves around the trigeminal–autonomic reflex.[1] There is considerable experimental animal literature to document that stimulation of trigeminal afferents can result in cranial autonomic outflow, the trigeminal–autonomic reflex.[102] In fact, some degree of cranial autonomic symptomatology is a normal physiologic response to cranial nociceptive input, and patients with other headache syndromes often report these symptoms.[2] The distinction between the TACs and other headache syndromes is the degree of cranial autonomic activation and not its presence.[103,104]
The cranial autonomic symptoms may be prominent in the TACs due to a central disinhibition of the trigeminal–autonomic reflex.[105] Supporting evidence is emerging from functional imaging studies: Positron emission tomography studies in CH[106] and PH,[107] and functional MRI studies in SUNCT syndrome[95,108,109] have demonstrated hypothalamic activation. Importantly, the involvement of posterior hypothalamic structures may account for the rhythmicity or periodicity that is such a hallmark of cluster headache. Hypothalamic activation is not seen in experimental trigeminal distribution head pain.[110] There are direct hypothalamic–trigeminal connections.[111] There is abundant evidence for a role of the hypothalamus in mediating anti-nociceptive[112,113] and autonomic responses.[114] In fact, there is direct evidence from animal experimental studies for hypothalamic activation when intracranial pain structures are activated.[115] Moreover, the hypothalamic peptides Orexin A and B can elicit pro-nociceptive and anti-nociceptive effects in the trigeminal system.[116] These data have led to the suggestion that the TACs are probably due to an abnormality in the hypothalamus with subsequent trigeminovascular and cranial autonomic activation.
An important consideration is that the different studies outlined above are unable to resolve the paramount question of whether the detected hypothalamic alterations are pathognomonic for TAC or whether they merely represent an epiphenomenon of different pain conditions in general. It has recently been argued that hypothalamic derangements may not be specific to TACs.[117] Hypothalamic activation and structural alterations are not exclusively observed in TAC but can also be found in other primary headache disorders, including migraine.[118] hemicrania continua[64] and hypnic headache.[119] Further research is certainly needed to definitively ascertain the pathophysiological basis of the TACs.
Conclusions
The TACs are a group of primary headache disorders characterized by unilateral head pain that occurs in association with ipsilateral cranial autonomic features. The TACs include CH, PH, SUNCT and, its close relative, SUNA. The underlying pathophysiology is purported to involve a role for neurons in the region of the posterior hypothalamus, although it remains unclear whether the derangements in this region are specific to the TACs or a nonspecific epiphenomenon. Clinically, the syndromes can be distinguished by the frequency of attacks of pain, the length of the attacks and very characteristic responses to medical therapy. The differentiation is important because the treatments are so distinct.
Competing Interests
There are no competing interests. GL has no disclosures. MSM serves on the advisory board for Allergan and St Jude Medical, and has received payment for the development of educational presentations from Allergan, Merck Sharp and Dohme Ltd. and Medtronic.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Goadsby PJ, Lipton RB. A review of paroxysmal hemicranias, SUNCT syndrome and other short-lasting headaches with autonomic feature, including new cases. Brain. 1997;120:193–209. doi: 10.1093/brain/120.1.193. [DOI] [PubMed] [Google Scholar]
- 2.Benoliel R, Sharav Y. Trigeminal neuralgia with lacrimation or SUNCT syndrome? Cephalalgia. 1998;18:85–90. doi: 10.1046/j.1468-2982.1998.1802085.x. [DOI] [PubMed] [Google Scholar]
- 3.Tonon C, Guttmann S, Volpini M, Naccarato S, Cortelli P, D’Alessandro R. Prevalence and incidence of cluster headache in the Republic of San Marino. Neurology. 2002;58:1407–9. doi: 10.1212/wnl.58.9.1407. [DOI] [PubMed] [Google Scholar]
- 4.Torelli P, Beghi E, Manzoni GC. Cluster headache prevalence in the Italian general population. Neurology. 2005;64:469–74. doi: 10.1212/01.WNL.0000150901.47293.BC. [DOI] [PubMed] [Google Scholar]
- 5.Bahra A, May A, Goadsby PJ. Cluster headache: A prospective clinical study with diagnostic implications. Neurology. 2002;58:354–61. doi: 10.1212/wnl.58.3.354. [DOI] [PubMed] [Google Scholar]
- 6.Schurks M, Kurth T, de Jesus J, Jonjic M, Rosskopf D, Diener HC. Cluster headache: Clinical presentation, lifestyle features, and medical treatment. Headache. 2006;46:1246–54. doi: 10.1111/j.1526-4610.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 7.Rozen TD. Cluster headache with aura. Curr Pain Headache Rep. 2011;15:98–100. doi: 10.1007/s11916-010-0168-9. [DOI] [PubMed] [Google Scholar]
- 8.The International Classification of Headache Disorders: 2nd ed. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 9.Cittadini E, Matharu MS. Symptomatic trigeminal autonomic cephalalgias. Neurologist. 2009;15:305–12. doi: 10.1097/NRL.0b013e3181ad8d67. [DOI] [PubMed] [Google Scholar]
- 10.Levy MJ, Matharu MS, Meeran K, Powell M, Goadsby PJ. The clinical characteristics of headache in patients with pituitary tumours. Brain. 2005;128:1921–30. doi: 10.1093/brain/awh525. [DOI] [PubMed] [Google Scholar]
- 11.Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas: A systematic review. Cancer. 2004;101:613–9. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 12.Ekbom K, Monstad I, Prusinski A, Cole JA, Pilgrim AJ, Noronha D. Subcutaneous sumatriptan in the acute treatment of cluster headache: A dose comparison study. The Sumatriptan Cluster Headache Study Group. Acta Neurol Scand. 1993;88:63–9. doi: 10.1111/j.1600-0404.1993.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 13.Ekbom K, Waldenlind E, Cole J, Pilgrim A, Kirkham A. Sumatriptan in chronic cluster headache: Results of continuous treatment for eleven months. Cephalalgia. 1992;12:254–6. doi: 10.1046/j.1468-2982.1992.1204254.x. [DOI] [PubMed] [Google Scholar]
- 14.Gobel H, Lindner V, Heinze A, Ribbat M, Deuschl G. Acute therapy for cluster headache with sumatriptan: Findings of a one-year long-term study. Neurology. 1998;51:908–11. doi: 10.1212/wnl.51.3.908. [DOI] [PubMed] [Google Scholar]
- 15.Kudrow L. Response of cluster headache attacks to oxygen inhalation. Headache. 1981;21:1–4. doi: 10.1111/j.1526-4610.1981.hed2101001.x. [DOI] [PubMed] [Google Scholar]
- 16.Fogan L. Treatment of cluster headache. A double-blind comparison of oxygen v air inhalation. Arch Neurol. 1985;42:362–3. doi: 10.1001/archneur.1985.04060040072015. [DOI] [PubMed] [Google Scholar]
- 17.Cohen AS, Burns B, Goadsby PJ. High-flow oxygen for treatment of cluster headache: A randomized trial. JAMA. 2009;302:2451–7. doi: 10.1001/jama.2009.1855. [DOI] [PubMed] [Google Scholar]
- 18.van Vliet JA, Bahra A, Martin V, Ramadan N, Aurora SK, Mathew NT, et al. Intranasal sumatriptan in cluster headache: Randomized placebo-controlled double-blind study. Neurology. 2003;60:630–3. doi: 10.1212/01.wnl.0000046589.45855.30. [DOI] [PubMed] [Google Scholar]
- 19.Cittadini E, May A, Straube A, Evers S, Bussone G, Goadsby PJ. Effectiveness of intranasal zolmitriptan in acute cluster headache: A randomized, placebo-controlled, double-blind crossover study. Arch Neurol. 2006;63:1537–42. doi: 10.1001/archneur.63.11.nct60002. [DOI] [PubMed] [Google Scholar]
- 20.Rapoport AM, Mathew NT, Silberstein SD, Dodick D, Tepper SJ, Sheftell FD, et al. Zolmitriptan nasal spray in the acute treatment of cluster headache: A double-blind study. Neurology. 2007;69:821–6. doi: 10.1212/01.wnl.0000267886.85210.37. [DOI] [PubMed] [Google Scholar]
- 21.Robbins L. Intranasal lidocaine for cluster headache. Headache. 1995;35:83–4. doi: 10.1111/j.1526-4610.1995.hed3502083.x. [DOI] [PubMed] [Google Scholar]
- 22.Costa A, Pucci E, Antonaci F, Sances G, Granella F, Broich G, et al. The effect of intranasal cocaine and lidocaine on nitroglycerin-induced attacks in cluster headache. Cephalalgia. 2000;20:85–91. doi: 10.1046/j.1468-2982.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 23.Andersson PG, Jespersen LT. Dihydroergotamine nasal spray in the treatment of attacks of cluster headache. A double-blind trial versus placebo. Cephalalgia. 1986;6:51–4. doi: 10.1046/j.1468-2982.1986.0601051.x. [DOI] [PubMed] [Google Scholar]
- 24.Aurora SK, Silberstein SD, Kori SH, Tepper SJ, Borland SW, Wang M, et al. MAP0004, orally inhaled DHE: A randomized, controlled study in the acute treatment of migraine. Headache. 2011;51:507–17. doi: 10.1111/j.1526-4610.2011.01869.x. [DOI] [PubMed] [Google Scholar]
- 25.Kudrow L. Cluster Headache: Mechanisms and Management. Oxford, New York: Oxford University Press; 1980. [Google Scholar]
- 26.Couch JR, Jr, Ziegler DK. Prednisone therapy for cluster headache. Headache. 1978;18:219–21. doi: 10.1111/j.1526-4610.1978.hed1804219.x. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro RE. Corticosteroid treatment in cluster headache: Evidence, rationale, and practice. Curr Pain Headache Rep. 2005;9:126–31. doi: 10.1007/s11916-005-0050-3. [DOI] [PubMed] [Google Scholar]
- 28.Ambrosini A, Vandenheede M, Rossi P, Aloj F, Sauli E, Pierelli F, et al. Suboccipital injection with a mixture of rapid- and long-acting steroids in cluster headache: A double-blind placebo-controlled study. Pain. 2005;118:92–6. doi: 10.1016/j.pain.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Francis GJ, Becker WJ, Pringsheim TM. Acute and preventive pharmacologic treatment of cluster headache. Neurology. 2010;75:463–73. doi: 10.1212/WNL.0b013e3181eb58c8. [DOI] [PubMed] [Google Scholar]
- 30.Mather PJ, Silberstein SD, Schulman EA, Hopkins MM. The treatment of cluster headache with repetitive intravenous dihydroergotamine. Headache. 1991;31:525–32. doi: 10.1111/j.1526-4610.1991.hed3108525.x. [DOI] [PubMed] [Google Scholar]
- 31.Magnoux E, Zlotnik G. Outpatient intravenous dihydroergotamine for refractory cluster headache. Headache. 2004;44:249–55. doi: 10.1111/j.1526-4610.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 32.May A, Leone M, Afra J, Linde M, Sandor PS, Evers S, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13:1066–77. doi: 10.1111/j.1468-1331.2006.01566.x. [DOI] [PubMed] [Google Scholar]
- 33.Bussone G, Leone M, Peccarisi C, Micieli G, Granella F, Magri M, et al. Double blind comparison of lithium and verapamil in cluster headache prophylaxis. Headache. 1990;30:411–7. doi: 10.1111/j.1526-4610.1990.hed3007411.x. [DOI] [PubMed] [Google Scholar]
- 34.Leone M, D’Amico D, Frediani F, Moschiano F, Grazzi L, Attanasio A, et al. Verapamil in the prophylaxis of episodic cluster headache: A double-blind study versus placebo. Neurology. 2000;54:1382–5. doi: 10.1212/wnl.54.6.1382. [DOI] [PubMed] [Google Scholar]
- 35.Matharu MS, Goadsby PJ. Trigeminal autonomic cephalgias. J Neurol Neurosurg Psychiatry. 2002;72(Suppl 2):ii19–26. doi: 10.1136/jnnp.72.suppl_2.ii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekbom K. Lithium for cluster headache: Review of the literature and preliminary results of long-term treatment. Headache. 1981;21:132–9. doi: 10.1111/j.1526-4610.1981.hed2104132.x. [DOI] [PubMed] [Google Scholar]
- 37.Wheeler SD, Carrazana EJ. Topiramate-treated cluster headache. Neurology. 1999;53:234–6. doi: 10.1212/wnl.53.1.234. [DOI] [PubMed] [Google Scholar]
- 38.Forderreuther S, Mayer M, Straube A. Treatment of cluster headache with topiramate: Effects and side-effects in five patients. Cephalagia. 2002;22:186–9. doi: 10.1046/j.1468-2982.2002.00339.x. [DOI] [PubMed] [Google Scholar]
- 39.Lainez MJ, Pascual J, Pascual AM, Santonja JM, Ponz A, Salvador A. Topiramate in the prophylactic treatment of cluster headache. Headache. 2003;43:784–9. doi: 10.1046/j.1526-4610.2003.03137.x. [DOI] [PubMed] [Google Scholar]
- 40.Mathew NT, Kailasam J, Meadors L. Prophylaxis of migraine, transformed migraine, and cluster headache with topiramate. Headache. 2002;42:796–803. doi: 10.1046/j.1526-4610.2002.02183.x. [DOI] [PubMed] [Google Scholar]
- 41.Leone M, Dodick D, Rigamonti A, D’Amico D, Grazzi L, Mea E, et al. Topiramate in cluster headache prophylaxis: An open trial. Cephalalgia. 2003;23:1001–2. doi: 10.1046/j.1468-2982.2003.00665.x. [DOI] [PubMed] [Google Scholar]
- 42.Dodick DW, Capobianco DJ. Treatment and management of cluster headache. Curr Pain Headache Rep. 2001;5:83–91. doi: 10.1007/s11916-001-0015-0. [DOI] [PubMed] [Google Scholar]
- 43.Leone M, D’Amico D, Moschiano F, Fraschini F, Bussone G. Melatonin versus placebo in the prophylaxis of cluster headache: A double-blind pilot study with parallel groups. Cephalalgia. 1996;16:494–6. doi: 10.1046/j.1468-2982.1996.1607494.x. [DOI] [PubMed] [Google Scholar]
- 44.Peres MF, Rozen TD. Melatonin in the preventive treatment of chronic cluster headache. Cephalalgia. 2001;21:993–5. doi: 10.1046/j.1468-2982.2001.00307.x. [DOI] [PubMed] [Google Scholar]
- 45.Leandri M, Luzzani M, Cruccu G, Gottlieb A. Drug.resistant cluster headache responding to gabapentin: A pilot study. Cephalalgia. 2001;21:744–6. doi: 10.1046/j.1468-2982.2001.00260.x. [DOI] [PubMed] [Google Scholar]
- 46.Hering R, Kuritzky A. Sodium valproate in the treatment of cluster headache: An open clinical trial. Cephalalgia. 1989;9:195–8. doi: 10.1046/j.1468-2982.1989.0903195.x. [DOI] [PubMed] [Google Scholar]
- 47.Gallagher RM, Mueller LL, Freitag FG. Divalproex sodium in the treatment of migraine and cluster headaches. J Am Osteopath Assoc. 2002;102:92–4. [PubMed] [Google Scholar]
- 48.El Amrani M, Massiou H, Bousser M. A negative trial of sodium valproate in cluster headache: Methodological issues. Cephalalgia. 2002;22:205–8. doi: 10.1046/j.1468-2982.2002.00349.x. [DOI] [PubMed] [Google Scholar]
- 49.Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick D. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26:1168–70. doi: 10.1111/j.1468-2982.2006.01173.x. [DOI] [PubMed] [Google Scholar]
- 50.Jarrar RG, Black DF, Dodick DW, Davis DH. Outcome of trigeminal nerve section in the treatment of chronic cluster headache. Neurology. 2003;60:1360–2. doi: 10.1212/01.wnl.0000055902.23139.16. [DOI] [PubMed] [Google Scholar]
- 51.Mathew NT, Hurt W. Percutaneous radiofrequency trigeminal gangliorhizolysis in intractable cluster headache. Headache. 1988;28:328–31. doi: 10.1111/j.1526-4610.1988.hed2805328.x. [DOI] [PubMed] [Google Scholar]
- 52.Magis D, Allena M, Bolla M, De Pasqua V, Remacle JM, Schoenen J. Occipital nerve stimulation for drug-resistant chronic cluster headache: A prospective pilot study. Lancet Neurol. 2007;6:314–21. doi: 10.1016/S1474-4422(07)70058-3. [DOI] [PubMed] [Google Scholar]
- 53.Burns B, Watkins L, Goadsby PJ. Treatment of intractable chronic cluster headache by occipital nerve stimulation in 14 patients. Neurology. 2009;72:341–5. doi: 10.1212/01.wnl.0000341279.17344.c9. [DOI] [PubMed] [Google Scholar]
- 54.Leone M, Franzini A, Broggi G, Bussone G. Hypothalamic stimulation for intractable cluster headache: Long-term experience. Neurology. 2006;67:150–2. doi: 10.1212/01.wnl.0000223319.56699.8a. [DOI] [PubMed] [Google Scholar]
- 55.Schoenen J, Di Clemente L, Vandenheede M, Fumal A, De Pasqua V, Mouchamps M, et al. Hypothalamic stimulation in chronic cluster headache: A pilot study of efficacy and mode of action. Brain. 2005;128:940–7. doi: 10.1093/brain/awh411. [DOI] [PubMed] [Google Scholar]
- 56.D’Andrea G, Nordera GP, Piacentino M. Effectiveness of hypothalamic stimulation in two patients affected by intractable chronic cluster headache. Neurology. 2006;5(Suppl 2):140. [Google Scholar]
- 57.Leone M. Deep brain stimulation in headache. Lancet Neurol. 2006;5:873–7. doi: 10.1016/S1474-4422(06)70575-0. [DOI] [PubMed] [Google Scholar]
- 58.Fontaine D, Lazorthes Y, Mertens P, Blond S, Geraud G, Fabre N, et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: A randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain. 2010;11:23–31. doi: 10.1007/s10194-009-0169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cittadini E, Matharu MS, Goadsby PJ. Paroxysmal hemicrania: A prospective clinical study of 31 cases. Brain. 2008;131:1142–55. doi: 10.1093/brain/awn010. [DOI] [PubMed] [Google Scholar]
- 60.Boes CJ, Dodick DW. Refining the clinical spectrum of chronic paroxysmal hemicrania: A review of 74 patients. Headache. 2002;42:699–708. doi: 10.1046/j.1526-4610.2002.02171.x. [DOI] [PubMed] [Google Scholar]
- 61.Antonaci F, Sjaastad O. Chronic paroxysmal hemicrania (CPH): A review of the clinical manifestations. Headache. 1989;29:648–56. doi: 10.1111/j.1526-4610.1989.hed2910648.x. [DOI] [PubMed] [Google Scholar]
- 62.Matharu MS, Boes CJ, Goadsby PJ. Management of trigeminal autonomic cephalgias and hemicrania continua. Drugs. 2003;63:1637–77. doi: 10.2165/00003495-200363160-00002. [DOI] [PubMed] [Google Scholar]
- 63.Antonaci F, Pareja JA, Caminero AB, Sjaastad O. Chronic paroxysmal hemicrania and hemicrania continua. Parenteral indomethacin: The ‘indotest’. Headache. 1998;38:122–8. doi: 10.1046/j.1526-4610.1998.3802122.x. [DOI] [PubMed] [Google Scholar]
- 64.Matharu MS, Cohen AS, McGonigle DJ, Ward N, Frackowiak RS, Goadsby PJ. Posterior hypothalamic and brainstem activation in hemicrania continua. Headache. 2004;44:747–61. doi: 10.1111/j.1526-4610.2004.04141.x. [DOI] [PubMed] [Google Scholar]
- 65.Lisotto C, Maggioni F, Mainardi F, Zanchin G. Rofecoxib for the treatment of chronic paroxysmal hemicrania. Cephalalgia. 2003;23:318–20. doi: 10.1046/j.1468-2982.2003.00500.x. [DOI] [PubMed] [Google Scholar]
- 66.Chakravarty A, Mukherjee A, Roy D. Trigeminal autonomic cephalgias and variants: Clinical profile in Indian patients. Cephalalgia. 2004;24:859–66. doi: 10.1111/j.1468-2982.2004.00759.x. [DOI] [PubMed] [Google Scholar]
- 67.Siow HC. Seasonal episodic paroxysmal hemicrania responding to cyclooxygenase-2 inhibitors. Cephalalgia. 2004;24:414–5. doi: 10.1111/j.1468-2982.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 68.Mathew NT, Kailasam J, Fischer A. Responsiveness to celecoxib in chronic paroxysmal hemicrania. Neurology. 2000;55:316. doi: 10.1212/wnl.55.2.316. [DOI] [PubMed] [Google Scholar]
- 69.Lenzer J. FDA advisers warn: COX 2 inhibitors increase risk of heart attack and stroke. Br Med J. 2005;330:440. doi: 10.1136/bmj.330.7489.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen AS, Goadsby PJ. Paroxysmal hemicrania responding to topiramate. J Neurol Neurosurg Psychiatry. 2007;78:96–7. doi: 10.1136/jnnp.2006.096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Afridi SK, Shields KG, Bhola R, Goadsby PJ. Greater occipital nerve injection in primary headache syndromes–prolonged effects from a single injection. Pain. 2006;122:126–9. doi: 10.1016/j.pain.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 72.Rossi P, Di Lorenzo G, Faroni J, Sauli E. Seasonal, extratrigeminal, episodic paroxysmal hemicrania successfully treated with single suboccipital steroid injections. Eur J Neurol. 2005;12:903–6. doi: 10.1111/j.1468-1331.2005.01106.x. [DOI] [PubMed] [Google Scholar]
- 73.Williams MH, Broadley SA. SUNCT and SUNA: Clinical features and medical treatment. J Clin Neurosci. 2008;15:526–34. doi: 10.1016/j.jocn.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Cohen AS, Matharu MS, Goadsby PJ. Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) or cranial autonomic features (SUNA)–a prospective clinical study of SUNCT and SUNA. Brain. 2006;129:2746–60. doi: 10.1093/brain/awl202. [DOI] [PubMed] [Google Scholar]
- 75.Matharu MS, Cohen AS, Boes CJ, Goadsby PJ. Short-lasting unilateral neuralgiform headache with conjunctival injection and tearing syndrome: A review. Curr Pain Headache Rep. 2003;7:308–18. doi: 10.1007/s11916-003-0052-y. [DOI] [PubMed] [Google Scholar]
- 76.Pareja JA, Sjaastad O. SUNCT syndrome. A clinical review. Headache. 1997;37:195–202. doi: 10.1046/j.1526-4610.1997.3704195.x. [DOI] [PubMed] [Google Scholar]
- 77.Matharu MS, Levy MJ, Merry RT, Goadsby PJ. SUNCT syndrome secondary to prolactinoma. J Neurol Neurosurg Psychiatry. 2003;74:1590–2. doi: 10.1136/jnnp.74.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jimenez Caballero PE. SUNCT syndrome in a patient with prolactinoma and cabergoline-induced attacks. Cephalalgia. 2007;27:76–8. doi: 10.1111/j.1468-2982.2007.01229.x. [DOI] [PubMed] [Google Scholar]
- 79.Matharu MS, Cohen AS, Goadsby PJ. SUNCT syndrome responsive to intravenous lidocaine. Cephalalgia. 2004;24:985–92. doi: 10.1111/j.1468-2982.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 80.Cohen AS. Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing. Cephalalgia. 2007;27:824–32. doi: 10.1111/j.1468-2982.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- 81.Cohen AS, Matharu MS, Goadsby PJ. Suggested guidelines for treating SUNCT and SUNA. Cephalalgia. 2005;25:1200. [Google Scholar]
- 82.Pareja JA, Caballero V, Sjaastad O. SUNCT syndrome. Statuslike pattern. Headache. 1996;36:622–4. doi: 10.1046/j.1526-4610.1996.3610622.x. [DOI] [PubMed] [Google Scholar]
- 83.D’Andrea G, Granella F, Ghiotto N, Nappi G. Lamotrigine in the treatment of SUNCT syndrome. Neurology. 2001;57:1723–5. doi: 10.1212/wnl.57.9.1723. [DOI] [PubMed] [Google Scholar]
- 84.Rossi P, Cesarino F, Faroni J, Malpezzi MG, Sandrini G, Nappi G. SUNCT syndrome successfully treated with topiramate: Case reports. Cephalalgia. 2003;23:998–1000. doi: 10.1046/j.1468-2982.2003.00566.x. [DOI] [PubMed] [Google Scholar]
- 85.Graff-Radford SB. SUNCT syndrome responsive to gabapentin. Cephalalgia. 2000;20:515–7. doi: 10.1046/j.1468-2982.2000.00065.x. [DOI] [PubMed] [Google Scholar]
- 86.Hunt CH, Dodick DW, Bosch P. SUNCT responsive to gabapentin. Headache. 2002;42:525–6. doi: 10.1046/j.1526-4610.2002.02129.x. [DOI] [PubMed] [Google Scholar]
- 87.Porta-Etessam J, Martinez-Salio A, Berbel A, Benito-Leon J. Gabapentin (neurontin) in the treatment of SUNCT syndrome. Cephalalgia. 2002;22:249. doi: 10.1046/j.1468-2982.2002.00375.x. [DOI] [PubMed] [Google Scholar]
- 88.Morales-Asin F, Espada F, Lopez-Obarrio LA, Navas I, Escalza I, Iniguez C. A SUNCT case with response to surgical treatment. Cephalalgia. 2000;20:67–8. doi: 10.1046/j.1468-2982.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- 89.Matharu MS, Cohen AS, Goadsby PJ. SUNCT syndrome responsive to intravenous lidocaine. Cephalalgia. 2004;24:985–92. doi: 10.1111/j.1468-2982.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 90.Hannerz J, Linderoth B. Neurosurgical treatment of short-lasting, unilateral, neuralgiform hemicrania with conjunctival injection and tearing. Br J Neurosurg. 2002;16:55–8. doi: 10.1080/026886902753512600. [DOI] [PubMed] [Google Scholar]
- 91.Effendi K, Jarjoura S, Mathieu D. SUNCT syndrome successfully treated by gamma knife radiosurgery: Case report. Cephalalgia. 2011;31:870–3. doi: 10.1177/0333102411404716. [DOI] [PubMed] [Google Scholar]
- 92.Black DF, Dodick DW. Two cases of medically and surgically intractable SUNCT: A reason for caution and an argument for a central mechanism. Cephalalgia. 2002;22:201–4. doi: 10.1046/j.1468-2982.2002.00348.x. [DOI] [PubMed] [Google Scholar]
- 93.Gardella L, Viruega A, Rojas H, Nagel J. A case of a patient with SUNCT syndrome treated with Jannetta procedure. Cephalalgia. 2001;21:996–9. doi: 10.1046/j.1468-2982.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 94.Lagares A, Gomez PA, Perez-Nunez A, Lobato RD, Ramos A. Short-lasting unilateral neuralgiform headache with conjunctival injection and tearing syndrome treated with microvascular decompression of the trigeminal nerve: Case report. Neurosurgery. 2005;56:E413. doi: 10.1227/01.neu.0000147981.90703.8f. [DOI] [PubMed] [Google Scholar]
- 95.Sprenger T, Valet M, Platzer S, Pfaffenrath V, Steude U, Tolle TR. SUNCT: Bilateral hypothalamic activation during headache attacks and resolving of symptoms after trigeminal decompression. Pain. 2005;113:422–6. doi: 10.1016/j.pain.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 96.Chaila E, Ali E, Rawluk D, Hutchinson M. ‘Switching off’ SUNCT by sudden head movement: A new symptom. J Neurol. 2011;258:694–5. doi: 10.1007/s00415-010-5804-0. [DOI] [PubMed] [Google Scholar]
- 97.Williams M, Bazina R, Tan L, Rice H, Broadley SA. Microvascular decompression of the trigeminal nerve in the treatment of SUNCT and SUNA. J Neurol Neurosurg Psychiatry. 2010;81:992–6. doi: 10.1136/jnnp.2009.182824. [DOI] [PubMed] [Google Scholar]
- 98.Matharu M, Watkins L, Shanahan P. Treatment of medically intractable SUNCT and SUNA with occipital nerve stimulation. J Neurol Neurosurg Psychiatry. 2010;81:e51–2. [Google Scholar]
- 99.May A, Bahra A, Buchel C, Turner R, Goadsby PJ. Functional magnetic resonance imaging in spontaneous attacks of SUNCT: Short-lasting neuralgiform headache with conjunctival injection and tearing. Ann Neurol. 1999;46:791–4. doi: 10.1002/1531-8249(199911)46:5<791::aid-ana18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 100.Leone M, Franzini A, D’Andrea G, Broggi G, Casucci G, Bussone G. Deep brain stimulation to relieve drug-resistant SUNCT. Ann Neurol. 2005;57:924–7. doi: 10.1002/ana.20507. [DOI] [PubMed] [Google Scholar]
- 101.Lyons MK, Dodick DW, Evidente VG. Responsiveness of short-lasting unilateral neuralgiform headache with conjunctival injection and tearing to hypothalamic deep brain stimulation. J Neurosurg. 2009;110:279–81. doi: 10.3171/2008.4.17493. [DOI] [PubMed] [Google Scholar]
- 102.May A, Goadsby PJ. The trigeminovascular system in humans: Pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab. 1999;19:115–27. doi: 10.1097/00004647-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 103.Goadsby PJ, Matharu MS, Boes CJ. SUNCT syndrome or trigeminal neuralgia with lacrimation. Cephalalgia. 2001;21:82–3. doi: 10.1046/j.1468-2982.2001.00175.x. [DOI] [PubMed] [Google Scholar]
- 104.Goadsby PJ. Trigeminal autonomic cephalalgias: Fancy term or constructive change to the IHS classification? J Neurol Neurosurg Psychiatry. 2005;76:301–5. doi: 10.1136/jnnp.2004.036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leone M, Bussone G. Pathophysiology of trigeminal autonomic cephalalgias. Lancet Neurol. 2009;8:755–64. doi: 10.1016/S1474-4422(09)70133-4. [DOI] [PubMed] [Google Scholar]
- 106.May A, Bahra A, Buchel C, Frackowiak RS, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352:275–8. doi: 10.1016/S0140-6736(98)02470-2. [DOI] [PubMed] [Google Scholar]
- 107.Matharu MS, Cohen AS, Frackowiak RS, Goadsby PJ. Posterior hypothalamic activation in paroxysmal hemicrania. Ann Neurol. 2006;59:535–45. doi: 10.1002/ana.20763. [DOI] [PubMed] [Google Scholar]
- 108.May A, Bahra A, Buchel C, Turner R, Goadsby PJ. Functional magnetic resonance imaging in spontaneous attacks of SUNCT: Short-lasting neuralgiform headache with conjunctival injection and tearing. Ann Neurol. 1999;46:791–4. doi: 10.1002/1531-8249(199911)46:5<791::aid-ana18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 109.Cohen A, Matharu M, Kalisch R, Friston K, Goadsby P. Functional MRI in SUNCT shows differential hypothalamic activation with increasing pain. Cephalalgia. 2004;24:1098–9. [Google Scholar]
- 110.May A. New insights into headache: An update on functional and structural imaging findings. Nat Rev Neurol. 2009;5:199–209. doi: 10.1038/nrneurol.2009.28. [DOI] [PubMed] [Google Scholar]
- 111.Malick A, Burstein R. Cells of origin of the trigeminohypothalamic tract in the rat. J Comp Neurol. 1998;400:125–44. doi: 10.1002/(sici)1096-9861(19981012)400:1<125::aid-cne9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 112.Wang Q, Mao LM, Han JS. Naloxone-reversible analgesia produced by microstimulation of the arcuate nucleus of the hypothalamus in pentobarbital-anesthetized rats. Exp Brain Res. 1990;80:201–4. doi: 10.1007/BF00228862. [DOI] [PubMed] [Google Scholar]
- 113.Dafny N, Dong WQ, Prieto-Gomez C, Reyes-Vazquez C, Stanford J, Qiao JT. Lateral hypothalamus: Site involved in pain modulation. Neuroscience. 1996;70:449–60. doi: 10.1016/0306-4522(95)00358-4. [DOI] [PubMed] [Google Scholar]
- 114.Lumb BM, Lovick TA. The rostral hypothalamus: An area for the integration of autonomic and sensory responsiveness. J Neurophysiol. 1993;70:1570–7. doi: 10.1152/jn.1993.70.4.1570. [DOI] [PubMed] [Google Scholar]
- 115.Benjamin L, Levy MJ, Lasalandra MP, Knight YE, Akerman S, Classey JD, et al. Hypothalamic activation after stimulation of the superior sagittal sinus in the cat: A Fos study. Neurobiol Dis. 2004;16:500–5. doi: 10.1016/j.nbd.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 116.Bartsch T, Levy MJ, Knight YE, Goadsby PJ. Differential modulation of nociceptive dural input to [hypocretin] orexin A and B receptor activation in the posterior hypothalamic area. Pain. 2004;109:367–78. doi: 10.1016/j.pain.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 117.Holle D, Katsarava Z, Obermann M. The hypothalamus: Specific or nonspecific role in the pathophysiology of trigeminal autonomic cephalalgias? Curr Pain Headache Rep. 2011;15:101–7. doi: 10.1007/s11916-010-0166-y. [DOI] [PubMed] [Google Scholar]
- 118.Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47:1418–26. doi: 10.1111/j.1526-4610.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 119.Holle D, Naegel S, Krebs S, Gaul C, Gizewski E, Diener HC, et al. Hypothalamic gray matter volume loss in hypnic headache. Ann Neurol. 2011;69:533–9. doi: 10.1002/ana.22188. [DOI] [PubMed] [Google Scholar]


