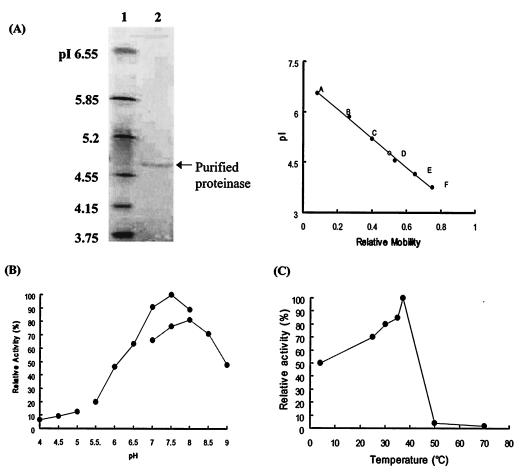
FIG. 2.
Characterization of the enzyme purified from C. neoformans culture filtrates. (A) Isoelectric focusing and pI determination. The isoelectric focusing gel range was pI 3 to 10. Lane 1, standard pI markers; lane 2, purified enzyme. The pI value of the purified enzyme was determined by the mobility of the purified enzyme relative to the standard pI markers. pI markers: A, human carbonic anhydrase B (pI 6.55); B, bovine carbonic anhydrase B (pI 5.85); C, β-lactaglobuin A (pI 5.2); D, soybean trypsin inhibitor (pI 4.55); E, glucose oxidase mannitol (pI 4.15); F, methyl red (dye) (pI 3.75). (B) pH dependence of the purified enzyme. The purified enzyme was incubated with azoalbumin in various pH buffer solutions. The maximal activity, seen in 0.1 M phosphate buffer (pH 7.5), is taken as 100%. Enzyme activity increased linearly from pH 6.0 to 7.5, and 80% of the total activity was exhibited between pH 7.0 and 8.0. (C) Temperature dependence of the purified proteinase. Ten microliters of purified enzyme was incubated in 290 μl of 0.1 M sodium phosphate buffer (pH 7.5) and 100 μl of azoalbumin substrate solution at 4, 25, 30, 35, 37, 50, and 70°C for 16 h. The proteolytic activity remaining was measured at OD440 by spectrophotometer.