Abstract
RNA interference (RNAi) provides an important tool for gene function discovery. It has been widely exploited in Caenorhabditis elegans ageing research because it does not appear to have any non-specific effects on ageing-related traits in that model organism. We show here that ubiquitous, adult-onset activation of the RNAi machinery, achieved by expressing a double stranded RNA targeting GFP or lacZ for degradation, or by increasing expression of Dicer substantially reduces lifespan in Drosophila melanogaster. Induction of GFPRNAi construct also alters the response of lifespan to nutrition, exacerbating the lifespan-shortening effects of food containing a high quantity of yeast. Our study indicates that activation of the RNAi machinery may have sequence-independent side-effects on lifespan, and that caution needs to be exercised when employing ubiquitous RNAi in Drosophila ageing studies. However, we also show that RNAi restricted to certain tissues may not be detrimental to lifespan.
Introduction
Ageing and the associated functional decline are a major medical and socioeconomic concern for developed countries [1], [2]. Despite the complexity of the process, we now known that ageing can be modulated by pharmacological, genetic and environmental interventions [2]. This has resulted in an explosion in research activity aimed at identifying the genes, pathways and processes that can be used as potential targets for interventions into human ageing, to achieve better health for older people. These efforts have been successful in identifying numerous longevity determinants, especially in the simpler, genetically-amenable animal models such as the nematode worm Caenorhabditis elegans and the fruit fly Drosophila melanogaster.
One of the main tools used for knocking-down gene activity in C. elegans ageing research is RNA interference (RNAi). RNAi is an evolutionarily conserved phenomenon where the activity of a gene is silenced post-transcriptionally by means of mRNA degradation triggered by a short, double-stranded RNA (dsRNA) complementary in sequence to a part of the target message [3]. The capacity for RNAi is native to the cell and utilises its inherent processing and degradation machinery, including the endoribonuclease Dicer, that are shared with other pathways, such as the microRNA (miRNA) processing machinery [3], [4]. Importantly, RNAi can be triggered by exogenously-provided dsRNA and for this reason it has been widely exploited for gene-function analysis [3].
In worms, RNAi can be achieved by simply feeding them Escherichia coli expressing a suitable dsRNA [3]. RNAi has been so extensively used in C. elegans ageing research that even genome-wide RNAi screens for longevity have been performed, where RNAi knock-down of certain genes has been found to extend lifespan [5], [6]. Other studies have used RNAi to determine the genetic requirements for lifespan extension by environmental changes, such as dietary restriction [7].
In stark contrast, ubiquitous expression of an RNAi construct has rarely been reported as extending lifespan in Drosophila, with some notable exceptions (eg. see reference [8]), whereas loss of function mutants, or induction of dominant negative forms of proteins often do extend lifespan [9], [10], [11], [12]. The general paucity of reports of lifespan-extension by ubiquitous RNAi in Drosophila could be attributable to a correlated negative effect stemming from the activation of the RNAi machinery, coupled with a publication bias for negative results. Such a deleterious side-effect of RNAi could mask any beneficial effects resulting from the loss-of-function of the targeted gene. Indeed, here we report that ubiquitous expression of dsRNAs targeting a portion of the GFP or lacZ gene, or induction of Dicer, results in shortened lifespan in Drosophila. Furthermore, the expression of the same GFP RNAi construct changes the response of lifespan to diet. Our study reveals that induction of RNAi can have sequence-independent, detrimental effects in the fly, and highlights that caution needs to be exercised when using RNAi as a tool for longevity studies in Drosophila.
Results
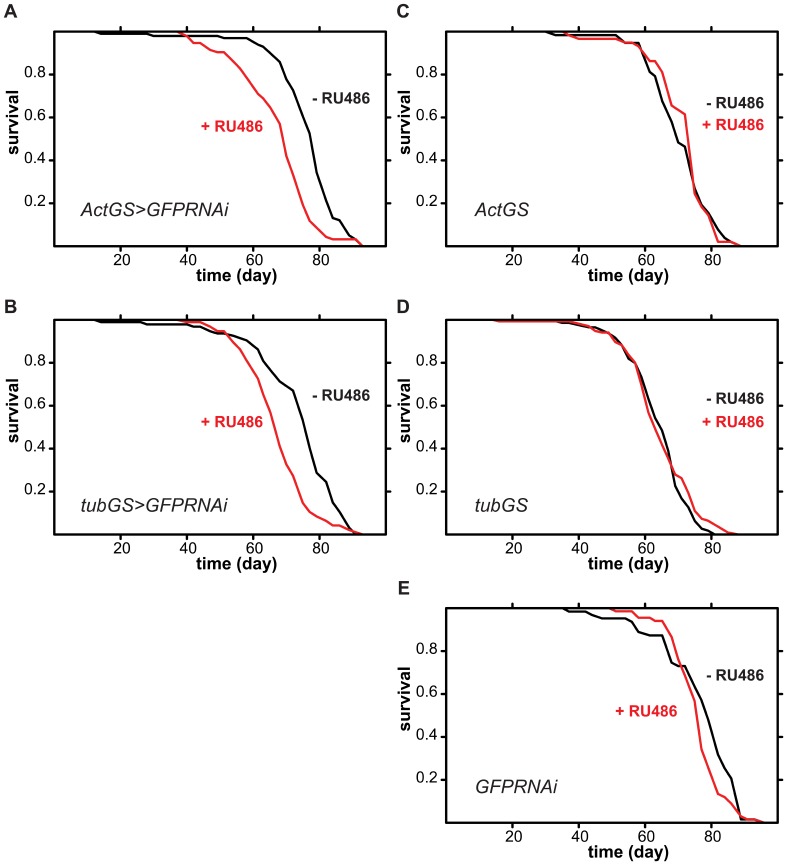
To examine the effects of activation of the RNAi machinery on Drosophila lifespan, we chose a construct that produces a dsRNA with no target mRNA in the fly, an inverted repeat of 604 bp of the GFP gene sequence (UAS-GFPRNAi), which can efficiently knock-down GFP expression [13]. To avoid confounding developmental effects, we used GeneSwitch drivers that are only induced upon feeding the flies the RU486 steroid drug [14], [15]. We focused on the effects in female flies since they are most commonly used in ageing studies. Driving the expression of the construct with two frequently used ubiquitous GeneSwitch drivers, actin GeneSwitch (ActGS) and tubulin GeneSwitch (tubGS) from day two of adulthood resulted in substantial and significant reduction in lifespan ( Figure 1A and B ). Feeding RU486 to control flies containing only the driver or only the transgene had no effect on their lifespan ( Figure 1C, D and E ). Hence activation of the RNAi machinery appears to decrease lifespan in Drosophila.
Figure 1. Ubiquitous adult-induced expression of dsRNA targeting GFP for RNAi shortens lifespan in Drosophila females.
Mated female flies of the indicated genotype were fed 1 SYA food containing RU486 (red lines) or not (black lines) from day two of adulthood. A and B Log-rank test detected significant differences between the induced and uninduced conditions (p<0.0001, n = ∼100 per condition). C, D and E Log-rank test did not detected significant differences between the induced and uninduced conditions (p>0.05, n = ∼100 per condition).
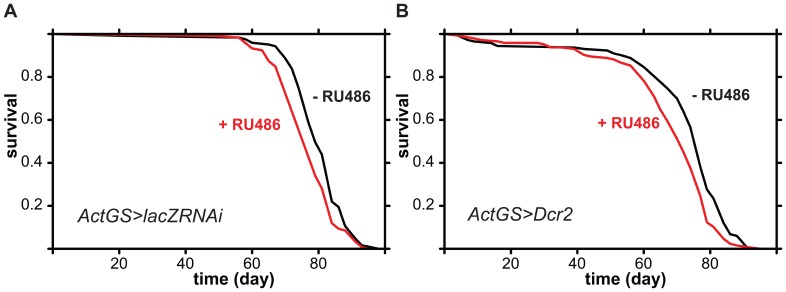
We examined the sequence of the GFPRNAi construct with the dsCheck program [16] and found that it does not contain any 19-mers that perfectly match to a part of the Drosphila genome. This indicated that the observed detrimental effect on lifespan was sequence-independent. To confirm this, we ubiquitously expressed an RNAi construct targeting the bacterial lacZ transcript (UAS-lacZRNAi) with the ActGS driver ( Figure 2A ), as well as activated the RNAi machinery not by inducing an exogenous dsRNA but by induction of Dicer, an endoribonuclease encoded by the Dcr2 gene ( Figure 2B ). Both these manipulations shortened lifespan, confirming that the reduction in lifespan results from sequence-independent effects of the activation of the RNAi machinery.
Figure 2. Ubiquitous adult-induced expression of dsRNA targeting lacZ for RNAi, or of Dicer2, shortens lifespan in Drosophila females.
A Mated ActGS>lacZRNAi female flies of the indicated genotype were fed 1 SYA food containing RU486 (red lines) or not (black lines) from day two of adulthood. Log-rank test detected significant differences between the induced and uninduced conditions (p = 0.004, n = ∼120 per condition). B Same as in A but for ActGS>Dcr2. Log-rank test detected significant differences between the induced and uninduced conditions (p = 0.0006, n = ∼150 per condition).
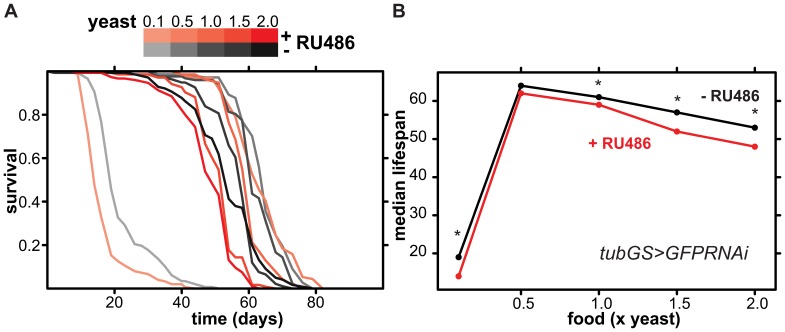
RNAi has also been used in the worm to test if particular genes are involved in mediating the effects of dietary restriction, a phenomenon where lifespan is maximised at intermediate food concentrations [7]. In Drosophila, dietary restriction can be achieved by dilution of the yeast component of fly food [17]. To assess if RNAi affected the response of the flies to dietary restriction, we investigated whether the flies subjected to RNAi differed from controls in their lifespan response. Ubiquitous expression of the GFPRNAi construct with the tubGS driver exacerbated the detrimental effect of RNAi on lifespan at higher and lower levels of yeast in the food, so that the two acted synergistically to shorten lifespan ( Figure 3A and B ). RNAi did not significantly shorten lifespan at its peak (0.5× yeast) but was detrimental at higher and lower yeast concentrations ( Figure 3B ), including the 1× yeast concentration that was tested above ( Figure 1 ). Analysing the data with Cox Proportional Hazard analysis [18] confirmed this, and both the effect of food or transgene induction were significant and so was their interaction (p<0.0001). Hence, activation of the RNAi machinery appears to alter the lifespan response to different yeast concentrations in Drosophila.
Figure 3. Lifespan-shortening effects of ubiquitous adult-induced expression of UAS-GFPRNAi are diet-dependent.
Mated female tubGS>GFPRNAi flies were reared on 1 SYA food and placed on different foods containing increasing amounts of yeast (0.1× to 2× relative to standard food) either containing RU486 (shades of red) or not (shades of grey) from day two of adulthood. A The resulting lifespans. B The median survival times, with asterisks indicating significant differences in survival between the induced (+ RU486) and uninduced (-RU486) conditions (Log-rank test, p<0.001, n = ∼150 per condition). The survival between induced and uninduced at 0.5× yeast was not significant (Log-rank test, p>0.5, n = ∼150 per condition). Cox Proportional Hazards analysis revealed significant effect of yeast quantity, presence of RU486 and their interaction (p<0.0001).
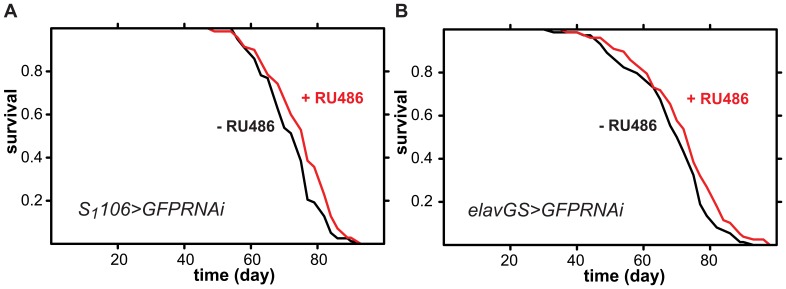
In Drosophila RNAi is potentially useful for tissue-restricted loss-of-function studies, so we wanted to examine if this, also, is necessarily detrimental to Drosophila lifespan. To assess this, we tested two GeneSwitch drivers with tissue-restricted expression that are commonly used in ageing studies: S1106, which drives expression in the midgut and abdominal fat body, and elavGS, which drives expression in neuronal cells [19]. Driving the expression of the GFPRNAi construct with either driver had no negative effect on lifespan ( Figure 4A and B ). Hence, localised activation of the RNAi machinery does not necessarily have a detrimental effect on lifespan. However, driving GFPRNAi with S1106 showed a slight but significant increase in survival ( Figure 4A ), indicating that caution will need to be exercised when employing tissue-specific drivers as well, on a case-by-case basis.
Figure 4. Adult-induced expression of dsRNA targeting GFP for RNAi in midgut and abdominal fat body or in neurons does not shorten lifespan in Drosophila females.
Mated female flies of indicated genotype were fed 1 SYA food containing RU486 (red lines) or not (black lines) from day two of adulthood. A Log-rank test detected a slight but significant increase in lifespan in the induced compared to the uninduced condition (p = 0.04, n = ∼80 per condition). B Log-rank test did not detected significant differences between the induced and uninduced conditions (p>0.05, n = ∼80 per condition).
Discussion
We show here that ubiquitously driving a construct expressing dsRNA containing a portion of the GFP or LacZ protein-coding sequence, or ubiquitous induction of Dicer, has a negative effect on Drosophila lifespan. Several unwanted effects of exogenously induced RNAi are known. Sequence-specific off-target events can be observed in both mammals and flies where, for example, the presence of short sequences homologous to a gene non-intentionally targeted by the construct can result in its degradation [20]. In Drosophila, the occurrence of these sequence-dependent off target effects correlates with the presence of 19mers in the dsRNA that are complementary to a non-intentionally targeted mRNA, resulting in its degradation [21]. Since the GFPRNAi construct contains no such 19mers and the negative effect on lifespan can be recapitulated using RNAi against lacZ or by Dicer over-expression, this reduction in lifespan is unlikely to result from sequence-dependent, off-target effects; rather it reveals that activation of the RNAi machinery may have sequence-independent, negative effects on Drosophila lifespan. At least two such sequence-independent side effects are documented in mammals. Firstly, dsRNA of certain lengths can result in activation of the interferon or Tol-like receptor response [20]. Secondly, high levels of expression of short hairpin RNAs used for RNAi can result in depletion of the components of the processing machinery, severely compromising cellular functions and causing organ damage in vivo [22]. In particular, high levels of expression of short hairpin RNAs in mouse liver have been shown to result in damage to liver tissue and ultimately to death of the mouse. This effect appears to be mediated by depletion of the dsRNA processing machinery away from its normal cellular roles such as processing of miRNAs [22]. A recent report has revealed the important role of miRNAs in Drosophila ageing [23] and so a similar phenomenon may underlie the lifespan shortening effect of RNAi in Drosophila.
Importantly, our study reveals that caution needs to exercised when using RNAi for lifespan assays in Drosophila and that, in addition to the sequence-specific off-target effects, sequence-independent effects also have to be controlled for. In particular, future studies will require employment of a non-specific RNAi construct, such as UAS-GFPRNAi, as a control treatment.
Materials And Methods
The UAS-GFPRNAi construct [13] and UAS-Dcr2 were obtained from the Bloomington stock center, tubGS (reported in [24]) was a kind gift of Scott Pletcher and ActGS [25] was a gift of John Tower. S1106 and elavGS have been described [19], [26]. All of the above constructs were backcrossed at least 6 times into a Dahomey background carrying a w1118 mutation and that had been cured of Wolbachia infection and frequently out-crossed to the Dahomey population housed in a population cage. UAS-lacZRNAi construct [27] was a kind gift of Martin Junger and Hugo Stocker. This construct was not backcrossed but the ActGS>lacZRNAi flies were created in a hybrid background using the backcrossed ActGS virgins and the lifespan of resulting progeny determined on food containing or not RU486. Stocks were maintained and experiments conducted at 25°C on a 12 h:12 h light:dark cycle at constant humidity using standard sugar/yeast medium (1 SYA) [28]. The food contained 5% sucrose (w/v), 1.5% agar (w/v), 0.3% propionic acid (v/v), 0.3% nipagen (w/v) and either 1% (0.1× yeast), 5% (0.5× yeast), 10% (1× yeast) or 20% (2× yeast) autolysed brewer's yeast (w/v) and was prepared as previously described [26]. The food with 1× yeast is referred to as 1 SYA. For lifespan assays, females were separated from males on day 2 of adulthood, transferred to food containing zero or 200 µM RU486, housed 10 per vial and transferred to new food 3 times per week. Statistical analysis was performed in JMP (version 9) software (SAS Institute) or Excel (Microsoft).
Acknowledgments
We thank Scott Pletcher, John Tower, Martin Junger and Hugo Stocker for fly lines.
Funding Statement
This work was funded by the Wellcome Trust and Max Planck (L.P.) and Excellence in Life Sciences (EMBO) and Marie Currie Fellowships (N.A.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Christensen K, Doblhammer G, Rau R, Vaupel JW (2009) Ageing populations: the challenges ahead. Lancet 374: 1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fontana L, Partridge L, Longo VD (2010) Extending healthy life span–from yeast to humans. Science 328: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hannon GJ (2002) RNA interference. Nature 418: 244–251. [DOI] [PubMed] [Google Scholar]
- 4. Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 5. Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, et al. (2003) A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet 33: 40–48. [DOI] [PubMed] [Google Scholar]
- 6. Hamilton B, Dong Y, Shindo M, Liu W, Odell I, et al. (2005) A systematic RNAi screen for longevity genes in C. elegans. Genes Dev 19: 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A (2007) PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 447: 550–555. [DOI] [PubMed] [Google Scholar]
- 8. Copeland JM, Cho J, Lo T Jr, Hur JH, Bahadorani S, et al. (2009) Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol 19: 1591–1598. [DOI] [PubMed] [Google Scholar]
- 9. Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, et al. (2001) A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292: 107–110. [DOI] [PubMed] [Google Scholar]
- 10. Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, et al. (2001) Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292: 104–106. [DOI] [PubMed] [Google Scholar]
- 11. Ikeya T, Broughton S, Alic N, Grandison R, Partridge L (2009) The endosymbiont Wolbachia increases insulin/IGF-like signalling in Drosophila. Proc Biol Sci 276: 3799–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slack C, Giannakou ME, Foley A, Goss M, Partridge L (2011) dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging Cell 10: 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roignant JY, Carre C, Mugat B, Szymczak D, Lepesant JA, et al. (2003) Absence of transitive and systemic pathways allows cell-specific and isoform-specific RNAi in Drosophila. RNA 9: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osterwalder T, Yoon KS, White BH, Keshishian H (2001) A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A 98: 12596–12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roman G, Endo K, Zong L, Davis RL (2001) P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A 98: 12602–12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naito Y, Yamada T, Matsumiya T, Ui-Tei K, Saigo K, et al. (2005) dsCheck: highly sensitive off-target search software for double-stranded RNA-mediated RNA interference. Nucleic Acids Res 33: W589–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mair W, Piper MD, Partridge L (2005) Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol 3: e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cox DR (1972) Regression models and life-tables. J R Stat Soc Ser B Stat Methodol 34: 187–220. [Google Scholar]
- 19. Poirier L, Shane A, Zheng J, Seroude L (2008) Characterization of the Drosophila gene-switch system in aging studies: a cautionary tale. Aging Cell 7: 758–770. [DOI] [PubMed] [Google Scholar]
- 20. Moffat J, Reiling JH, Sabatini DM (2007) Off-target effects associated with long dsRNAs in Drosophila RNAi screens. Trends Pharmacol Sci 28: 149–151. [DOI] [PubMed] [Google Scholar]
- 21. Kulkarni MM, Booker M, Silver SJ, Friedman A, Hong P, et al. (2006) Evidence of off-target effects associated with long dsRNAs in Drosophila melanogaster cell-based assays. Nat Methods 3: 833–838. [DOI] [PubMed] [Google Scholar]
- 22. Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, et al. (2006) Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441: 537–541. [DOI] [PubMed] [Google Scholar]
- 23. Liu N, Landreh M, Cao K, Abe M, Hendriks GJ, et al. (2012) The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature 482: 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernandez-Ayala DJ, Sanz A, Vartiainen S, Kemppainen KK, Babusiak M, et al. (2009) Expression of the Ciona intestinalis alternative oxidase (AOX) in Drosophila complements defects in mitochondrial oxidative phosphorylation. Cell Metab 9: 449–460. [DOI] [PubMed] [Google Scholar]
- 25. Ford D, Hoe N, Landis GN, Tozer K, Luu A, et al. (2007) Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp Gerontol 42: 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, et al. (2004) Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science 305: 361. [DOI] [PubMed] [Google Scholar]
- 27. Bulow MH, Aebersold R, Pankratz MJ, Junger MA (2010) The Drosophila FoxA ortholog Fork head regulates growth and gene expression downstream of Target of rapamycin. PLoS One 5: e15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, et al. (2007) Optimization of dietary restriction protocols in Drosophila. J Gerontol A Biol Sci Med Sci 62: 1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]