Abstract
Retrospective review of presentation, treatment and outcome of male breast cancer in a tertiary cancer centre in eastern India. Data of 42 male breast cancer (MBC) patients, who presented between April, 2001 and March, 2008 were collected from institute records with respect to epidemiological characteristics, clinical and pathological parameters, treatment pattern and outcome. This series includes 42 patients with mean age of 56 years (range 31–78 years). MBC represented 1.1 % of all breast cancer. History of lump in the breast with duration ranging from 1 month to 4 years was the most common clinical presentation (80.95 %). Histopathology found infiltrating ductal carcinoma in 35 (83.33 %), followed by papillary carcinoma in 3 (7.14 %), undifferentiated carcinoma in 2 (4.76 %), mucinous carcinoma in 1 (2.38 %) and myxofibrosarcoma in 1 (2.38 %) patient. Hormone receptor (HR) study was performed on 29 patients. Twenty six (89.7 %) patients were hormone receptor positive in that 8 (27.6 %) were ER positive and 18 (62.1 %) were ER and PR positive. 3 (10.3 %) were hormone receptor negative.Axillary lymph node dissection was performed on 30 patients. Of those, 60 % were found to be positive (pN+) and 40 % were negative (pN-). Of the patients with invasive carcinoma 2.86 % were pathologic stage I, 37.14 % stage II, 42.86 % stage III and 17.14 % stage IV. Of the 35 treated patients, total 30 (85.71 %) patients underwent surgery. The surgery consisted of a modified radical mastectomy (MRM) 24 (80 %), radical mastectomy according to Halsted (RM) 6 (20 %). Adjuvant therapy i.e. Chemotherapy and Radiotherapy was administered to the patient based upon their stage. The standard treatment for all HR positive patients was administration of tamoxifen. Based upon the follow-up information (ranging from 17 month to 136 months), 4 (14.28 %) patients developed local recurrence over 4 to 26 months (mean17.5 months) and 5 patients developed distant metastasis over 24 to132 months (mean 78 months). Disease specific survival varied from 4 months to 132 months, with a mean of 56.75 months. Thirteen out of 28 evaluable patients (46.43 %) were disease free at 5 years. Male Breast cancer is a rare disease often ignored in the community, because of which it is seeks medical attention at advanced stage. Majority of MBC are found to be HR positive, hence hormonal therapy should to be strongly considered and multicentric prospective studies are needed to improve outcome.
Keywords: Male breast cancer, Carcinoma of male breast, Breast carcinoma
Introduction
The earliest recorded case of male breast cancer (MBC) is described in Edwin Smith papyrus from Egypt (3000–2500 BC); in modern times, John of Arderne is reported to be the first to identify this disease in a male patient [1]. Though the incidence is reported to be rising, the over-all incidence is low. Because of the relative rarity, randomized trials are not possible, and only one prospective study has been reported [2]. MBC shares many characteristics with breast cancer in females, but there are significant epidemiologic and biologic differences between them. The present study was undertaken to analyze the clinicopathological profile of MBC in a tertiary cancer centre in Eastern India.
Materials and Methods
This is a retrospective study of all male patients presenting with breast cancer in Cancer Centre Welfare Home & Research Institute, Thakurpukur, Kolkata between April 2001 and March 2008; data was recorded regarding patient history, presenting signs and symptoms, presence of risk factors (if any), tumour characteristics, details of pre-treatment assessment, treatment and patterns of failure. All clinically and image-wise operable patients underwent FNAC or core needle biopsy of the lesion. In metastatic setting, a core needle biopsy was preferred to ascertain hormone receptor status. All operable patients underwent Radical Mastectomy (RM) or Modified Radical Mastectomy (MRM). Adjuvant chemotherapy or radiotherapy protocols generally followed that for female breast cancer. Patients received hormone therapy, depending on their hormone receptor status. The patients with metastatic disease were offered chemotherapy and hormone therapy. Radiotherapy was administered as a palliative measure, where indicated. Disease free survival was calculated by Kaplan Meier survival analysis.
Results
During the study period, a total of 3738 patients of histo-pathologically confirmed breast cancer were registered, of which 3696 (98.9 %) were female and 42 (1.1 %) were male.
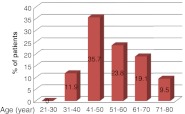
The mean age of male patients was 56 years, range being 30–78 years. The largest number was found in the age group of 41–50 years. (Fig. 1).
Fig. 1.
Age distribution of male breast cancer patients
The most common clinical presentation was mass (lump) in the breast, followed by ulceration. A mass in the breast and axillary lymphadenopathy were most common clinical findings (Table 1). The mass was right-sided in 22 (52.36 %) patients and left-sided in 20 (47.62 %) patients.
Table 1.
Clinical presentation of male breast cancer patients
| Clinical presentation | Number | Percentage |
|---|---|---|
| Mass (Lump) | 34 | 80.95 % |
| Skin ulceration | 6 | 14.29 % |
| Axillary swelling | 1 | 2.38 % |
| Unknown primary | 1 | 2.38 % |
| Axillary Lymphadenopathy | 18 | 42.85 % |
| Right sided lesion | 22 | 52.38 % |
| Left sided lesion | 20 | 47.62 % |
In the entire population, there was no family history of breast cancer or history of pre-existing conditions like gynaecomastia, testicular abnormality, liver cirrhosis, chronic renal insufficiency or any hormonal disorder.
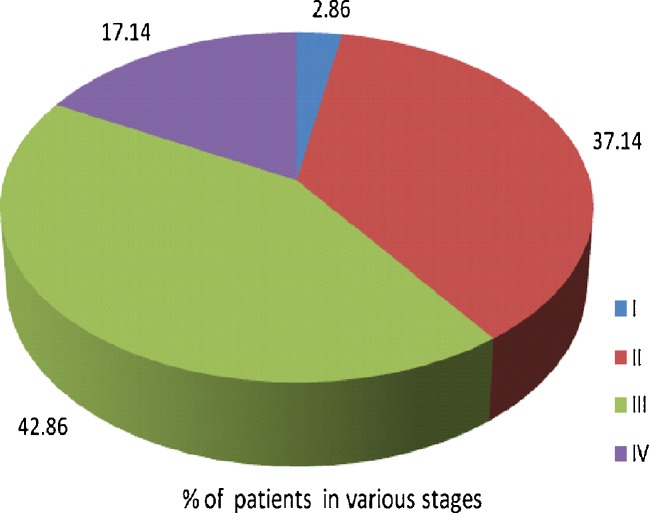
Of 42 MBC patients, 35 were completely staged, and data was not complete in the rest 7 patients. Thus, 37.14 % of the MBC patients presented with pathological stage II and 42.86 % with stage III disease (Table 2, Fig. 2).
Table 2.
Stage distribution in Male breast cancer patients
| Stage | TNM | Number | Total | Percentage |
|---|---|---|---|---|
| I | T1N0M0 | 1 | 1 | 2.86 % |
| IIA | T0N1M0 | 0 | 0 % | |
| T1N1M0 | 1 | 2.86 % | ||
| T2N0M0 | 3 | 8.57 % | ||
| IIB | T2N1M0 | 5 | 14.28 % | |
| T3N0M0 | 4 | 11.43 % | ||
| 9 | 25.23 % | |||
| II | 13 | 37.14 % | ||
| IIIA | T0N2M0 | 0 | 0 % | |
| T1N2M0 | 1 | 2.86 % | ||
| T2N2M0 | 0 | 0 % | ||
| T3N1Mo | 2 | 5.72 % | ||
| 3 | 8.57 % | |||
| IIIB | T4N0M0 | 3 | 8.57 % | |
| T4N1M0 | 4 | 11.43 % | ||
| T4N2M0 | 2 | 5.72 % | ||
| 9 | 25.7 % | |||
| IIIC | AnyT N3M0 | 3 | 8.57 % | |
| III | 15 | 42.86 % | ||
| IV | Any T, any N M1 | 6 | 6 | 17.14 % |
| 35 | 100 % |
Fig. 2.
Distribution of male breast cancer patients according to stage
Of the 42 patients in whom pathologic confirmation was available, infiltrating duct carcinoma was found in 35 (83.3 %), papillary carcinoma in 3 (7.1 %), undifferentiated carcinoma in 2 (4.76 %), mucinous carcinoma and myxofibrosarcoma 1 each (2.38 %). Hormonal status was available in 29 patients. Twenty six (89.7 %) patients were hormone receptor positive in that 8 (27.6 %) were ER positive and 18 patients (62.1 %) were ER and PR positive. Three (10.3 %) patients were hormone receptor negative.
Total thirty five patients received treatment. Thirty (85.71 %) patients underwent surgery from stage I to Stage III disease, 24 (80 %) modified radical mastectomy (MRM) and 6 (20 %) radical mastectomy (RM). Of all surgically treated, 28 patients received chemotherapy and radiotherapy. Five patients from stage IV disease received treatment. Two patients received chemotherapy on tamoxifen failure, 2 patients both chemotherapy and radiotherapy (palliative for bone metastases), and 1patient only radiotherapy (haemostatic, refused any other treatment). All hormone receptor positive patients, except the one who refused any treatment except haemostatic radiation, received tamoxifen as additional treatment after completion of surgery, chemotherapy and radiation.
Out of these 35 treated patients, 7 were lost to follow up. The rest have a follow up period varying from 17 months to 136 months (mean 63.2 months). The median duration of follow up was 54 months. Four patients were reported with local recurrence ranging from 4 months to 26 months (mean 17.5 months). Five patients developed distant metastasis (multiple site metastasis in 1 patient at 2 years, bone metastases in 2 patients at 9 and 11 years respectively, and lung metastases in 2 patients, both at 4 years). Disease-free survival varied from 4 months to 132 months (mean 56.75 months) (Fig. 3). Thirteen out of 28 evaluable patients (46.43 %) were disease free at 5 years. As the male breast cancer is a rare disease, the study population is small and also complete data on follow up of all patients is not available (For e.g., in Stage I, there is only one patient and in Stage IV, there are only six patients. More importantly, several patients had chosen not to follow up.). Therefore, it is statistically inappropriate (due to very few samples) and incomplete (due to loss of follow up) to do stage-wise survival analysis.
Fig. 3.
Kaplan-Meier disease free survival analysis of evaluable 28 patients after completion of treatment
Discussion
MBC is a rare disease. It accounts for 0.7 % of all breast cancers [3] and 0.17 % of all cancers in males [4]. It shows significant geographic variation. The incidence rate in Europe and in the US is 1 in 100,000, in Japan is <5 in 100,000, while in some parts of Africa it may be as high as 15 % of all breast cancers [5]. In India, the Age Standardized Incidence (ASR) of MBC was reported as 0.4 per 100,000 populations in Mumbai in 2000[6]. The mean age of diagnosis is 67 years in the US population, 5 years older than the mean age in women [7]. In the present series, the mean age at diagnosis in men was 56 years, which was about 10 years younger than reported in world literature [6]. The incidence rate is reported to be steadily rising till the age of 80 years, where it tends to plateau [6]. In the present series, however, the peak incidence was in mid-forties, with a declining trend thereafter.
Implicating factors: Factors that lead to changes in the endocrine milieu (Klinefelter’s syndrome, exogenous estrogen as in prostate carcinoma, chronic liver disease, obesity, or testicular hypofunction as in crypto-orchidism, orchiectomy or viral orchitis), family history of breast cancer, and genetic abnormalities such BRCA 1 and BRCA 2 have been implicated in the causation of MBC [6]. However, in the present series, no such factors have been identified (Genetic testing for BRCA 1 and BRCA 2 not done in present series).
Presenting features: Most common clinical presentation was Painless mass in the breast. Goss et al [8], in a retrospective study of 229 patients over a period of 40 years, reported that breast mass was the presenting feature in 85.6 % patient. In the present study, a mass was found in 80.95 % of patients. Other studies also report the incidence in the 80–90 % range [8–10]. Though, incidence as low as 13 % has also been reported [11]. A slight preponderance of left side over right side in a ratio of 1.07:1 has been reported [12]. In the present study, there was a right sided preponderance of 1.1:1 over the left side.
Stage at presentation: A report analyzing the NCI-SEER data [7] found the incidence of different stages at presentation as: stage 0–10 %, stage I–29 %, stage II–38 %, stage III–7 % and stage IV–8 %. In the present study, the stage-wise distribution showed a large difference, stage 0–0 %, stage I–2.86 %, stage II–37.14 %, stage III–42.86 %, and stage IV–17.14 %. In this study, the probable causes of increased incidence of MBC at advanced stages includes, patient’s ignorance and illiteracy, misdiagnosis during initial stages, patient’s low economic condition driving them to traditional medicine during early stages.
Hormone receptor status: More than 90 % of MBCs are hormone receptor positive [13, 14]. In this series, 89.7 % were hormone receptor positive, which is consistent with the previous literature.
Prognostic factors: Traditionally, MBC is considered to have worse over-all 5 year survival of 40–65 %, compared to 80 % in women [15]. However, when matched for stage, age and hormone receptor status, male and female breast cancer patients show the same over-all survival pattern [16]. Stage, nodal status (positivity and number of involved nodes), size and hormone receptor status are considered as important prognostic factors [12]. It has been suggested that the biology of MBC resembles late-onset type of breast cancer in females [17], and thus may reflect the prognostic features of that disease. The number of patients and their follow-up in the present series is not significant for statistical analysis.
Treatment of localized disease: Radical Mastectomy (RM) has been the procedure of choice in earlier series [18], because of the large mass size of the cancer at initial presentation. Present-days less extensive surgery is preferred such as modified radical mastectomy (MRM), which is oncologically equivalent RM [6]. In the present series, 30 (85.71 %) out of the 35 treated patients underwent surgery, MRM was performed on 24 (80 %) patients and RM was performed on 6 (20 %) patients, which is consistent with present recommendation.
Role of radiotherapy: Due to lack of controlled trial, the role of radiotherapy in an adjuvant setting is not well-defined. However reportedly, radiation reduces post-operative locoregional recurrence rate, especially when pectoral muscles/chest wall is involved, though does not improve disease-specific survival [19]. In the present series, since almost all operable patients had advanced local disease, radiation was administered to all of them. Of the 28 evaluable patients treated with surgery with curative intent, 4 (14.28 %) patients developed local recurrence. The postoperative locoregional recurrence rate without radiotherapy has been reported to vary from 4 to 31 % [20].
Role of chemotherapy: Like radiotherapy, the role of chemotherapy in MBC is also not well defined. MBC patients are less likely to receive adjuvant chemotherapy, because of their more advanced age, and more incidence of hormone positivity [21]. CMF is the only chemotherapy protocol to be prospectively studied in the adjuvant setting. The NCI MB-82 study reported a 42 % 20 year survival in a group of 31 node positive patients after 12 cycles of CMF [22]. A study from MD Anderson Cancer Centre reported that adriamycin-based chemotherapy was more frequently used than CMF (81 % versus 16 %), and also had a reduced risk of death [23]. In the present series, all 28 evaluable postoperative patients received chemotherapy; 20 patients received FAC/FEC as the preferred modality and 8 patients CMF (as second choice, because of medical/financial reasons). Though the numbers are small, the failure patterns in both groups are similar.
Role of hormone therapy: Tamoxifen is the preferred endocrine agent [24]. Goss et al [8] reported the positive effect of adjuvant tamoxifen in both actuarial 5 year survival and disease-free survival. In the present series, all 26 hormone receptor positive patients received 5 years of adjuvant tamoxifen therapy. The status of other hormonal agents (letrozole, anastrazole, and fulvestrant) is less defined and was not used in this series.
Treatment for metastatic disease: Hormone manipulation is the mainstay of management since the pioneering report from Farrow and Adair showing the positive impact of orchiectomy in metastatic MBC [25]. However, with the availability of hormonal agents, ablative measures like orchiectomy, adrenalectomy or hypophysectomy are no longer performed. Tamoxifen is the preferred first-line hormonal agent, with an objective response rate of over 80 % in receptor positive patients [24]. On failure, second-line hormonal may include orchiectomy or luteinizing hormone-releasing hormone agonists with or without antiandrogens [26]. Chemotherapy is indicated in receptor negative or non-responders [20]. In the present series, patients on tamoxifen failure were treated with chemotherapy. The role of radiotherapy in the metastatic setting is mainly for palliation of bone pains or for control of bleeding (which was the reason in the sole case in the present series).
Survival: 5 year disease-free survival has been reported to vary from 35 to 65 % [8, 27, 28]. In the present series, the 5 year disease-free survival was 46.43 %.
Conclusion
MBC is a relatively rare disease and the treatment is generally extrapolated from female breast cancer data. Multicentric large prospective data is lacking. In the present retrospective study, the experience of a tertiary institution in eastern India in dealing with MBC is presented. There is general conformity with the experience elsewhere, with some differences observed. Most large single-institution retrospective series span a long period of time, and clubbing them together is questionable. Multicentric multinational studies also suffer from inherent disparities amongst the patient population; hence zonal multicentric prospective studies are needed to improve prognosis and survival of male breast cancer patients.
References
- 1.Redlich PN, Donegan WL. Carcinoma of the breast in men. In: Winchester DJ, Winchester DP, Decker BC, editors. American Cancer Society Atlas of Clinical Oncology Breast Cancer. London: Inc Hamilton; 2000. pp. 239–252. [Google Scholar]
- 2.Bagley CS, Wesley MN, Young RC, et al. Adjuvant chemotherapy in males with cancer of the breast. Am J Clin Oncol. 1987;10:55–60. doi: 10.1097/00000421-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Giordano SH. A review of diagnosis and management of male breast cancer. Oncologist. 2005;10:471–479. doi: 10.1634/theoncologist.10-7-471. [DOI] [PubMed] [Google Scholar]
- 4.Applebaum AH, Evans GF, Levy KR, et al. Radiographics. 1999;19:559–568. doi: 10.1148/radiographics.19.3.g99ma01559. [DOI] [PubMed] [Google Scholar]
- 5.Field KM, Campbell B, Boer R. Male breast cancer: Progress, prognosis and future pathways. Asia Pac J ClinOncol. 2008;4:6–17. doi: 10.1111/j.1743-7563.2008.00141.x. [DOI] [Google Scholar]
- 6.Contractor KB, Kaur K, Rodrigues GS (2008) Male breast cancer: Is the scenario changing? World J Surg Oncol. doi:10.1186/1477-7819-6-58 [DOI] [PMC free article] [PubMed]
- 7.Giordano SH, Cohen DS, Buzdar AU, et al. Breast carcinoma in men: A population based study. Cancer. 2004;101:51–57. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 8.Goss PE, Reid C, Pintilie M, et al. Male breast carcinoma. Cancer. 1999;85:629–639. doi: 10.1002/(SICI)1097-0142(19990201)85:3<629::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 9.Erlichman C, Murphy KC, Elkahim T. Male breast cancer: A 13 year review of 89 patients. J ClinOncol. 1984;2:903–909. doi: 10.1200/JCO.1984.2.8.903. [DOI] [PubMed] [Google Scholar]
- 10.Schieke O, Agarwal A, Ayatunde AA, Rampaul R, Robertson JFR. Male breast cancer: A review of clinical management. Breast Canc Res Treat. 2007;103:11–21. doi: 10.1007/s10549-006-9356-z. [DOI] [PubMed] [Google Scholar]
- 11.Borgen PI, Wong GI, Vlamis V, et al. Current management of male breast cancer: A review of 104 cases. Ann Surg. 1992;215:451–459. doi: 10.1097/00000658-199205000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giordano SH, Buzdar AU, Hortobagyi GN, et al. Breast cancer in men. Ann Intern Med. 2002;137:678–687. doi: 10.7326/0003-4819-137-8-200210150-00013. [DOI] [PubMed] [Google Scholar]
- 13.Borgen PI, Senie RT, McKinnon WMP, et al. Carcinoma of the male breast: Analysis of prognosis compared to matched female patients. Ann Surg Oncol. 1997;4:385–389. doi: 10.1007/BF02305550. [DOI] [PubMed] [Google Scholar]
- 14.Doyen J, Italiano A, Largillier R, et al. Aromatase inhibition in male breast cancer patients: Biological and clinical implications. Ann Oncol. 2010;21:1243–1245. doi: 10.1093/annonc/mdp450. [DOI] [PubMed] [Google Scholar]
- 15.Hinrichs CS, Watroba NL, Rezaishiraz H, et al. Lymphedema secondary to postmastectomy radiation: Incidence and risk factors. Ann Surg Oncol. 2004;11:573–580. doi: 10.1245/ASO.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 16.El-Tamer MB, Komaneka IK, Troxel A. Men with breast cancer has better disease-specific survival than women. Arch Surg. 2004;139:1079–1082. doi: 10.1001/archsurg.139.10.1079. [DOI] [PubMed] [Google Scholar]
- 17.Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: A population based comparison with female breast cancer. J Clin Oncol. 2010;28:232–239. doi: 10.1200/JCO.2009.23.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komaneka IK, Miller KD, Sledge WG Jr (2006) Male breast cancer. In Raghavan D, Brecher ML, Johnson DH et al. (ed) Textbook of uncommon cancer, 3rd edn. John Wiley & Sons 201–208
- 19.Atahan L, Yidiz F, Selek U, et al. Perioperative radiotherapy in the treatment of male breast carcinoma; a single institution experience. J Natl Med Assoc. 2006;98:559–563. [PMC free article] [PubMed] [Google Scholar]
- 20.Vetto JT (2010) Breast diseases in males. In Jatoi I, Kaufmann M (ed) Management of breast diseases, Springer-Verlag. 471–496
- 21.Scott-Conner CE, Jochimsen PR, Menck HR, et al. An analysis of male and female breast cancer treatment and survival among demographically identical pairs of patients. Surgery. 1999;126:775–780. doi: 10.1016/S0039-6060(99)70135-2. [DOI] [PubMed] [Google Scholar]
- 22.Walshe JM, Berman AW, Vatas U, et al. A prospective study of adjuvant CMF in males with node positive breast cancer: 20 year follow-up. Breast Canc Res Treat. 2007;103:177–183. doi: 10.1007/s10549-006-9363-0. [DOI] [PubMed] [Google Scholar]
- 23.Giordino S, Perkins GH, Broglio K, et al. Adjuvant systemic therapy for male breast cancer. Cancer. 2005;104:2359–2364. doi: 10.1002/cncr.21526. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal A, Ayantunde AAA, Rampaul R, Robertson JFR. Male breast cance: A review of clinical management. Breast Canc Res Treat. 2007;103:11–21. doi: 10.1007/s10549-006-9356-z. [DOI] [PubMed] [Google Scholar]
- 25.Farrow J, Adair F. Effect of orchiectomy on skeletal metastases from cancer of the male breast. Science. 1942;95:654–657. doi: 10.1126/science.95.2478.654. [DOI] [PubMed] [Google Scholar]
- 26.Volm MD. Male breast cancer. Curr Treat Options Oncol. 2003;4:159–164. doi: 10.1007/s11864-003-0017-8. [DOI] [PubMed] [Google Scholar]
- 27.Vinod SK, Pendlebury SC. Cancer of the male breast: A review of adjuvant therapy. Australas Radiol. 1999;43:69–72. doi: 10.1046/j.1440-1673.1999.00601.x. [DOI] [PubMed] [Google Scholar]
- 28.Borgen PI, Wong GY, Vlamis V. Current management of male breast cancer: A review of 104 cases. Ann Surg. 1992;215:451–457. doi: 10.1097/00000658-199205000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]