Abstract
Recent studies suggest that drug addicted individuals have a dampened cortical response to non-drug rewards. However, it remains unclear whether recency of drug use impacts this impairment. Therefore, in this study, recency of cocaine use was objectively determined by measuring cocaine in urine on study day. Thirty-five individuals with current cocaine use disorder [CUD: 21 testing positive (CUD+) and 14 testing negative (CUD−) for cocaine in urine] and 23 healthy controls completed a sustained attention task with graded monetary incentives (0¢, 1¢ and 45¢). Unlike in controls, in both CUD subgroups P300 amplitude was not modulated by the varying amounts of money and the CUD− showed the most severe impairment as documented by the lowest P300 amplitudes and task accuracy. Moreover, while recency of drug use was associated with better accuracy and higher P300 amplitudes, chronic drug use was associated with lower sensitivity to money. These results extend our previous findings of decreased sustained sensitivity to monetary reward in CUD+ to recently abstaining individuals, where level of impairment was most severe. Taken together, these results support the self-medication hypothesis, where CUD may be self-administering cocaine to avoid or compensate for underlying cognitive and emotional difficulties albeit with a long-term detrimental effect on sensitivity to non-drug reward.
Keywords: abstinence, cocaine addiction, event-related potential (ERP), P300
1. Introduction
A typical pattern of drug addiction in humans is characterized by intermittent periods of abstinence from drug-taking followed by increased craving and relapse (Gawin, 1991; O'Brien, 1997). These stages in drug addiction are thought to result from enduring drug-induced neuroadaptations within the mesocorticolimbic dopaminergic reward circuitry although such neural compromises may also precede, and predispose to, the development of drug addiction (Goldstein and Volkow, 2002; Kalivas and McFarland, 2003; Shalev et al., 2002; Volkow et al., 2004). Irrespective of the direction of causality, dysregulated sensitivity to reward has been shown in drug addicted individuals. For example, using functional magnetic resonance imaging (fMRI), we reported a compromised cortical sensitivity to monetary reward on a sustained attention task in individuals with cocaine use disorders (CUD) (Goldstein et al., 2007a); similar results have since been reported in alcoholics (Chen et al., 2007) and pathological gamblers (de Ruiter et al., 2009).
In a validation study (Goldstein et al., 2008), we investigated the P300 event-related potential (ERP), shown to have a core role in processing the incentive value of reinforcers on select tasks in healthy individuals (Hajcak et al., 2005; Sato et al., 2005; Wu and Zhou, 2009), and reported that CUD failed to show the expected graded P300 response to different levels of monetary reward (i.e., 45¢ > 0¢) (Goldstein et al., 2008). In contrast to other ERP studies in CUD (Bauer, 2001; Biggins et al., 1997; Gooding et al., 2008), and in opioid (Papageorgiou et al., 2004), alcohol (Fein and Chang, 2006) and nicotine (Anokhin et al., 2000) dependent individuals, however, we did not observe overall reduced P300 amplitudes in our sample.
Variability in recency of drug use may have contributed to this inconsistency in the P300 results. While most other studies recruited abstaining subjects from treatment facilities (Bauer, 2001; Biggins et al., 1997; Gooding et al., 2008), we recruited subjects abstaining for less than 4 days prior to study day (Goldstein et al., 2008). Indeed, abstinence has been shown to impact several cognitive [learning, memory and executive functions (Woicik et al., 2009)] and emotional [sustained emotional reactivity to drug cues (Dunning et al., 2011)] functions that are essential for processing the incentive value of reinforcers.
Therefore, the goal of the current study was to investigate contribution of abstinence length in current cocaine users to the monetary reward-elicited P300 amplitudes. For this purpose, in addition to the 36 subjects [18 CUD; all positive for cocaine in urine (CUD+) and 18 controls] that were included in our previous report (Goldstein et al., 2008), CUD who were negative for cocaine in urine (CUD−) were also included. Note that all CUD used crack/cocaine in the past 30 days, and that inclusion of the CUD− subgroup is entirely new to this study.
Given results by others (Bauer, 2001; Biggins et al., 1997; Fein and Chang, 2006; Gooding et al., 2008; Papageorgiou et al., 2004) and our prior neuropsychological results where we reported that cognitive impairment was most severe in CUD− as compared to CUD+ (Woicik et al., 2009), we a priori hypothesized that the recently abstinent group (with less recent cocaine use) will manifest the most severe reward-related P300 impairments. In addition to the P300, we explored earlier ERP components relevant to our task: the fronto-central P200, that has recently been described in the context of task relevance, attention and motivational spillover (Carretie et al., 2001a; Franken et al., 2010; Holroyd and Coles, 2002; Holroyd et al., 2008; Potts, 2004; Potts et al., 2006), and the fronto-central N200 that has been associated with biologically significant events (Campanella et al., 2002), stimulus novelty (Daffner et al., 2000) and top-down cortical inhibition (Folstein and Van Petten, 2008). Analyses pertaining to these earlier components were exploratory.
2. Methods
2.1. Participants
Thirty-five CUD and 23 healthy controls were recruited through advertisements in local newspapers and by word-of-mouth; one CUD was recruited from a treatment facility. All subjects underwent full physical and neurological examinations by a neurologist and a diagnostic interview by a clinical psychologist. The interview included the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1996), the Addiction Severity Index (McLellan et al., 1992), the Cocaine Selective Severity Assessment Scale (Kampman et al., 1998), the Cocaine Craving Questionnaire (Tiffany et al., 1993) and the Severity of Dependence Scale (Gossop et al., 1992).
Subjects were further screened to exclude for (1) history of head trauma or loss of consciousness (> 30 min) or any other neurological disease; (2) abnormal vital signs at time of screening and history of major medical conditions; (3) history of major psychiatric disorder, including current dependence on or abuse of any substance [other than cocaine abuse or dependence for CUD and/or nicotine dependence for all subjects]; (4) severe levels of self-reported depression (Beck et al., 1996) (scores > 20); (5) history of gambling as measured with the South Oaks Gambling Scale (Lesieur and Blume, 1987) (scores > 5); (6) except for cocaine in the CUD subjects, positive urine screens for any other psychoactive drugs; and (7) current use of any medication, including psychotropic medications.
The recency of cocaine use was objectively indexed by urine screening on study day using the triage urine panel (Biopsych™, detects drug use within 72 hours of study). Results of this test divided our CUD sample into two subgroups: those who tested positive for cocaine (CUD+: N=21, 3 new subjects were added to our previous sample) and those who tested negative for cocaine (CUD−: N=14). All CUD used crack/cocaine in the past 30 days and met DSM-IV criteria for current cocaine dependence [N=29 (N=11 in CUD−)] or abuse [N=6 (N=3 in CUD−)]. As anticipated, the two CUD subgroups differed on frequency of recent (past 30 days) and more protracted (last 12 months) cocaine use and on duration of current abstinence, such that CUD+ reported more recent cocaine use and higher craving than the CUD− (Table 1). Study groups did not differ on distributions of gender, race, age, education, non-verbal intelligence (Wechsler, 1999), depression, and socioeconomic status (Table 1). However, group differences were observed for history of cigarette smoking (Table 1), and its potential contributions to results were inspected as reported in Analysis and Results. To avoid acute withdrawal effects, cigarette smokers were allowed to smoke cigarettes as usual. Subjects were fully informed of all study procedures and risks and provided written consent in accordance with the local Institutional Review Board.
Table 1.
Demographics and drug use-related measures of all study subjects
| Test (χ2, F, or Z) |
Control (N = 23) |
CUD+ (N = 21) |
CUD− (N = 14) |
|
|---|---|---|---|---|
| Demographics | ||||
| Gender: Male / Female | 1.0 | 15 / 8 | 16 / 5 | 11 / 3 |
| Race: African-American / Other | 6.3 | 13 / 10 | 18 / 3 | 13 / 1 |
| Age (years) | 1.4 | 40.7 ± 7.0 | 43.1 ± 6.1 | 43.9 ± 5.5 |
| Education (years) | 2.9 | 13.9 ± 1.9 | 13.0 ± 1.7 | 12.5 ± 2.1 |
| Non-Verbal IQ: Wechsler Abbreviated Scale of Intelligence : Matrix Reasoning Scale (Wechsler, 1999) | 1.9 | 10.8 ± 2.6 | 9.5 ± 3.3 | 11.3 ± 2.6 |
| Depression: Beck Depression Inventory II (Beck et al., 1996) | 1.4 | 3.7 ± 4.4 | 5.7 ± 4.0 | 4.0 ± 4.0 |
| Socioeconomic Status: Hollingshead Index | 2.8 | 35.5 ± 14.6 | 31.5 ± 11.3 | 25.4 ± 10.4 |
| Drug Use | ||||
| Cigarette Smokers (current or past / nonsmokers) | 26.5† | 6 / 17 | 18 / 3 | 12 / 2 |
| Daily cigarettes (current smokers: N = 3/17/10) | 0.4 | 6.0 ± 7.4 | 8.4 ± 7.4 | 6.8 ± 5.0 |
| Age of onset of cocaine (years) | −0.4 | -- | 24.2 ± 5.9 | 24.1 ± 7.3 |
| Duration of use of cocaine (years) | −0.5 | -- | 16.8 ± 6.2 | 16.1 ± 6.3 |
| Duration of current abstinence (days) | −3.8† | -- | 1.9 ± 1.6 | 7.2 ± 5.6 |
| Cocaine use during last 30 days: Days/week | −3.1† | -- | 4.3 ± 2.1 | 2.0 ± 1.7 |
| Cocaine use during last 30 days: $/sitting | −0.2 | -- | 85.3 ± 90.6 | 107.6 ± 105.9 |
| Cocaine use during last 12 months: Days/week | −2.4* | -- | 4.3 ± 2.3 | 2.4 ± 2.0 |
| Cocaine use during last 12 months: $/sitting | −1.1 | -- | 83.1 ± 78.0 | 151.3 ± 139.8 |
| Maximum cocaine use (Days/week) | −0.6 | -- | 5.9 ± 1.6 | 5.3 ± 2.5 |
| Total score on the Cocaine Selective Severity Assessment Scale (measure of withdrawal symptoms) (0–126) (Kampman et al., 1998) | −1.1 | -- | 16.7 ± 10.2 | 13.1 ± 9.5 |
| Severity of Dependence Scale (0–15) (Gossop et al., 1992) | −1.2 | -- | 6.7 ± 2.9 | 5.4 ± 4.3 |
| Cocaine Craving Questionnaire (0–45) (Tiffany et al., 1993b) | −2.4* | -- | 21.5 ± 10.9 | 11.9 ± 9.0 |
| Number of Rehabilitation attempts | −0.9 | -- | 1.40 ± 2.48 | 4.29 ± 9.44 |
p<.05;
p<.01;
Race: Other (Caucasian / Hispanic / Asian);
χ2 tests were used for categorical variables; Mann-Whitney U for all drug-related variables (continuous non-normally distributed variables) and ANOVAs for all comparisons between the three groups;
Values are frequencies or means ± standard deviation (SD).
2.2. Reward processing task
Subjects completed a monetary reward paradigm used previously in our laboratory (Goldstein et al., 2006; Goldstein et al., 2008), which included six sequences/blocks, each consisting of 9 “Go” and 9 “No-go” trials for each of three blocked monetary reward conditions: 45¢, 1¢, and 0¢, hence, 54 “Go” and 54 “No-go” trials per condition. Subjects were instructed to press a button upon seeing the target stimulus (S2) after a “Go” S1 stimulus and to refrain from pressing the button upon seeing S2 after a “No-go” S1 stimulus. Feedback was presented as $0.45, $0.01, $0.00 for correct responses/non-responses or an “X” for incorrect responses/non-responses (Figure 1). Details of the sustained reward task can be found in the supplementary material. As in the previous ERP study, subjects could receive up to $50 contingent on their task performance.
Figure 1.
Experimental paradigm for the monetary incentive task. Overall design and experimental conditions are depicted at the top; at each condition onset (conditions were separated by 30 s), a 5 s screen (not depicted) displayed the monetary reward (45¢, 1¢, 0¢). Together with the feedback delivered at the end of each trial, this 5 s screen (similar in appearance to the feedback screen) guaranteed the subjects were continuously aware of the reward contingencies. Inst. is Instruction. Resp. is Response.
2.3. Psychophysiological recording and behavioral measures
Electroencephalogram (EEG; Neuroscan Inc., Sterling USA) recordings were obtained using a 64 silver-silver chloride electrode cap positioned according to the International 10/20 system. Electrooculogram (EOG) electrodes at left supra- and infra-orbital sites and the right and left outer canthi recorded the blinks (and vertical eye movements) and horizontal eye movements, respectively. EEG recordings were sampled at 1000 Hz, band pass filtered at 0 – 70 Hz, while the electrode impedances were kept under 10 kΩ. Reaction time (RT) and performance accuracy were recorded during all task trials and conditions. At the end of the task, all subjects rated each monetary condition for its interest, excitement and frustration (see supplementary material).
2.4. EEG data reduction and analyses
The EEG was re-referenced to linked mastoid electrodes and divided into epochs extending from 200 msec before the onset of S1 to 1800 msec after. All epochs were then subjected to a baseline correction algorithm with respect to the 200 msec pre-stimulus baseline, band pass filtering (0.1 – 30 Hz) and artifact rejection procedures (automatically, to remove EOG-detected eye movements; and visually, to reject trials containing muscle artifacts and head movement). After artifact rejection, there was a minimum of 30 epochs per task condition. Separate averages were composed for each money condition (45¢, 1¢ and 0¢) for ‘Go’ trials only, chosen a priori as our previous study showed significant differences among the monetary conditions in ‘Go’ trials only (the ‘No-Go’ trials were not analyzed as our previous studies using the same task showed these trials were not modulated by money) (Goldstein et al., 2008).
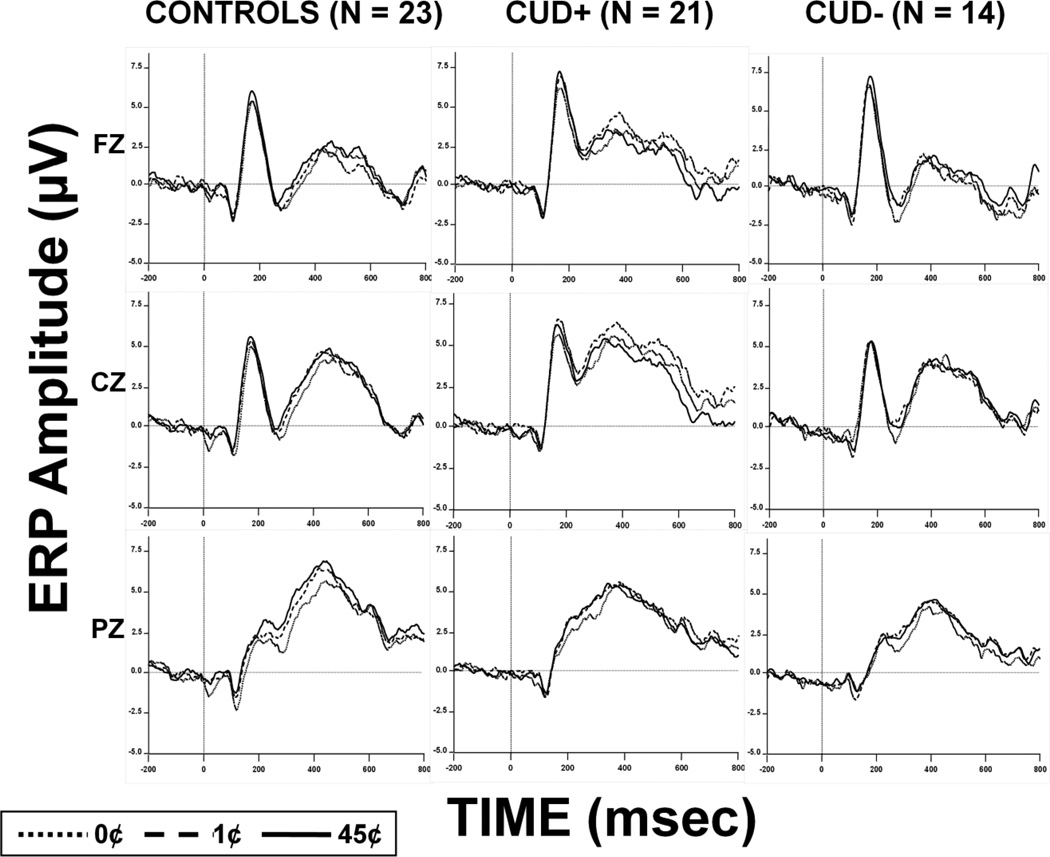
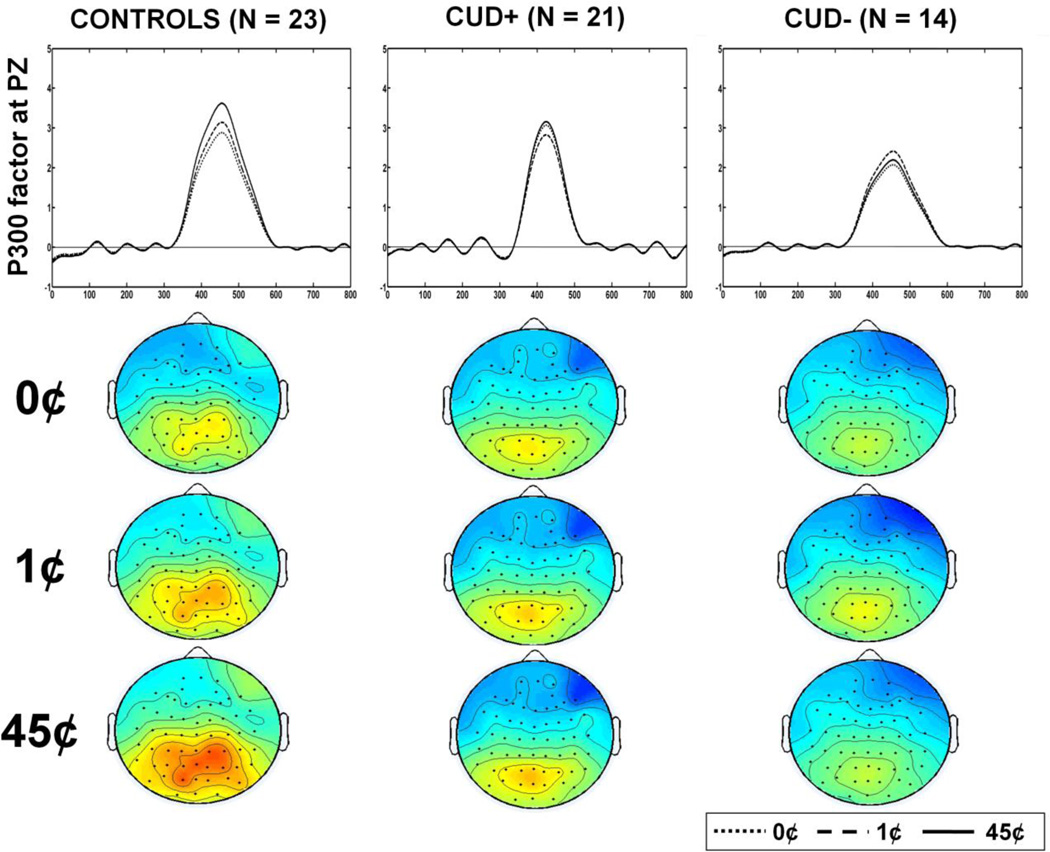
Given the pronounced nature of the P200 and N200 peaks, they were scored as the most positive and negative amplitudes in the 150 msec to 300 msec post-stimulus time window, respectively, at FZ and CZ electrodes (Bruin and Wijers, 2002; Franken et al., 2010; Nieuwenhuis et al., 2003; Potts et al., 1998). However, P300, being seemingly contaminated by other slow potentials, was isolated using temporal principal components analysis (PCA) [Matlab (Mathworks Inc., Natick, MA) based PCA Toolbox (version 2.23)] (Dien, 2010). Temporospatial PCA assesses variance across time and space (electrode sites) to maximize the separation of overlapping ERP components (Dien et al., 2005). For the temporal PCA step, we used all time points as variables and all subjects, monetary conditions, and all 64 electrodes as observations, whereas for the spatial PCA step, we used all 64 electrodes as variables and temporal PCA factors for all subjects and monetary conditions as observations. A temporosptial factor was identified as the P300 waveform based on its peak latency [occurring 250–600 msec after S1 (Picton, 1992; Pritchard, 1981)] and the scalp topography, with the maxima at parietal electrode sites (PZ) (Sutton et al., 1965). To account for variability in peak latency, a limitation of the temporospatial PCA (Curry et al., 1983), the average amplitude over ± 25 msec around the peak P300 component was used as the P300 amplitude for each subject and condition as previously recommended (Picton et al., 2000). The current analysis of P300 at PZ in response to correct trials is in accordance with previous studies (Goldstein et al., 2006; Goldstein et al., 2008; Hajcak et al., 2005; Yeung and Sanfey, 2004) and is also confirmed by quantitative methods [e.g., spatial PCA (Foti and Hajcak, 2009; Spencer et al., 2001)]. These grand-averages for each condition, group and selected electrodes can be inspected in Figure 2 and the P300 PCA factors and their scalp topographies can be inspected in Figure 3.
Figure 2.
Grand averaged ERP waveforms for control subjects (left; N=23), CUD+ (center; N=21) and CUD− (right; N=14) reflecting 0 msec to 800 msec after the cue stimulus (S1) for each monetary reward condition (45¢, 1¢, and 0¢) during the ‘Go’ trials at midline electrodes (FZ, CZ and PZ).
Figure 3.
The P300 factor isolated by the temporospatial PCA for the three study groups along with the scalp topographies for each monetary reward condition (45¢, 1¢, and 0¢).
The P200 and N200 amplitudes in response to correct trials were analyzed using a 2 [Electrode (FZ and CZ] × 3 [Money (45¢, 1¢ and 0¢)] × 3 [Group (Controls, CUD+ and CUD−)] repeated measures analysis of variance (ANOVA), while the PZ P300 amplitudes from the PCA component were analyzed using a 3 [Money (45¢, 1¢ and 0¢)] × 3 [Group (Controls, CUD+ and CUD−)] mixed ANOVA. Similarly to the P300 analyses, RT of all correct trials and task accuracy were analyzed using 3 × 3 mixed ANOVAs.
In all analyses, the Greenhouse-Geisser correction was applied for cases where Mauchly’s test showed the assumption of sphericity was not met. Significant effects were followed with post-hoc t-tests for ERP components and RT (normally distributed) and with the equivalent non-parametric tests (independent: Mann-Whitney U; or paired: Wilcoxon) for accuracy (not normally distributed). Cigarette smoking history, which differed significantly between the groups (Table 1), was covaried in subsequent ANCOVAs if it was significantly related to ERP and behavior variables. To test our a priori hypothesis (most severe impairment in sensitivity to monetary reward in CUD− compared to CUD+ and controls), planned between-group comparisons were conducted for P300 amplitude, even if the main effects or interactions with group were not significant. This practice, of reporting results of post-hoc tests even when main or interaction effects are not significant is recommended in case of strong a priori hypotheses (e.g., to avoid Type II error) (Ruxton and Beauchamp, 2008), and has been widely used especially in studies of populations that are difficult to recruit/engage (Larson et al., 2009; van Erp et al., 2002; Yang et al., 2009). Note that, given our prior ERP (Goldstein et al., 2008) and fMRI (Goldstein et al., 2007b) results, task accuracy and RT were similarly treated. All other effects were only followed with post-hoc analyses if main or interaction effects were significant.
Correlations between P300 amplitudes and RT and accuracy were also examined separately for all three monetary conditions and also for differential scores (45¢ minus 0¢, 45¢ minus 1¢, and 1¢ minus 0¢) for all subjects, and separately per study group. Finally, the correlations between the dependent measures (including the differential scores) and the drug use measures (listed in Table 1) were examined across all CUD and also for each CUD subgroup. To protect against Type I error, a significance level of p<0.01 was required for all correlations, while p<0.05 was reported as trend. In all correlation analyses, cigarette smoking history was controlled through partial correlations when it was associated with our dependent variables (Stevens, 1992).
3. Results
Cigarette smoking was not significantly associated (p>0.1) with any of our dependent variables and therefore will not receive further consideration in Results.
3.1. ERP results (Table 2 and Figure 2B)
Table 2.
The P200, N200, P300 Amplitudes and Behavioral (reaction time and accuracy) Dependent Variables for all Study Subjects as a Function of Group and Monetary Reward.
| P200 (µV) | N200 (µV) | P300 (µV) | Reaction Time (msec) |
Accuracy (%) |
||||
|---|---|---|---|---|---|---|---|---|
| FZ | CZ | FZ | CZ | PZ | ||||
|
Control (N=23) |
$0.00 | 7.08 (2.71) | 7.16 (2.42) | −3.41 (3.42) | −2.76 (3.76) | 3.05 (1.73) | 241.4 (21.8) | 93.1(5.8) |
| $0.01 | 7.23 (2.83) | 7.43 (2.66) | −3.28 (3.94) | −2.18 (4.40) | 3.52 (1.82) | 238.1 (20.2) | 92.8 (6.3) | |
| $0.45 | 7.89 (3.01) | 8.39 (2.77) | −3.46 (3.95) | −1.96 (4.36) | 3.99 (2.17) | 237.3 (22.6) | 93.4 (5.6) | |
|
CUD+ (N=21) |
$0.00 | 8.57 (3.80) | 8.56 (3.42) | −1.50 (4.20) | 0.28 (3.59) | 3.07 (1.86) | 235.2 (16.0) | 94.7 (11.1) |
| $0.01 | 9.54 (3.62) | 9.36 (2.93) | −0.72 (4.29) | 0.67 (3.54) | 2.85 (1.61) | 232.8 (14.6) | 95.5 (6.7) | |
| $0.45 | 9.20 (3.70) | 9.17 (3.48) | −0.55 (4.12) | 1.02 (3.51) | 2.99 (1.86) | 227.1 (15.0) | 95.3 (5.5) | |
|
CUD− (N=14) |
$0.00 | 7.72 (2.74) | 6.72 (2.46) | −3.50 (3.46) | −2.29 (3.28) | 2.14 (1.95) | 225.9 (18.0) | 87.5 (12.4) |
| $0.01 | 8.08 (3.33) | 7.07 (2.79) | −2.50 (3.95) | −0.99 (3.49) | 2.22 (2.01) | 219.5 (21.2) | 85.6 (11.1) | |
| $0.45 | 9.38 (3.94) | 7.63 (3.03) | −2.92 (3.28) | −1.83 (3.81) | 2.16 (1.79) | 221.4 (18.6) | 87.4 (9.7) | |
Mean (SD)
3.1.1. P200 results
The mixed ANOVA did not reveal significant group [F(2,55)=2.0, p=0.14] and electrode [F(1,55)=1.9, p=0.18] main effects, although there was a significant money main effect [F(2,54)=5.85, p=0.005]. Post-hoc paired t-tests showed this money main effect to be driven by significantly higher P200 amplitude for 45¢ condition as compared to 0¢ condition across all subjects [FZ: t(57)=2.8, p=0.007; CZ: t(57)=3.5, p=0.001]. None of the interaction effects reached significance (p>0.1). Thus, P200 was higher for reward vs. non-reward across all study groups at both frontal and central midline electrodes.
3.1.2. N200 results
All three main effects were significant: group [F(2,54)=3.4, p=0.04], money [F(2,54)=4.4, p=0.017], and electrode [F(1,55)=23.5, p<0.0001], while none of the interaction effects reached significance (p>0.1). Post-hoc t-tests revealed these main effects to be driven, respectively, by more negative N200 waveforms in controls compared to CUD+ (p=0.046), non-reward compared to reward [0¢>45¢: p=0.08; 0¢>1¢: p=0.02], and at FZ compared to CZ (p<0.0001) across all groups and money conditions. Thus, N200 was greatest in control subjects and in the non-reward task condition at FZ.
3.1.3. P300 results
Results of the 3 × 3 mixed ANOVA revealed a significant money main effect [45¢>0¢, F(2,54)=3.12, p=0.05] and money by group interaction [F(4,110)=4.06, p=0.004], whereas the group main effect did not reach significance [F(2,55)=2.46, p=0.10]. Post-hoc paired T-tests showed that the money main effect was driven entirely by differential responsiveness to money in controls [45¢=1¢>0¢, t(22)<−3.59, p<0.02], but not CUD+ [t(20)<1.59, p>0.1] or CUD− [t(13)<−1.25, p>0.2] or the combined CUD group [t(34)<1.1, p>0.2], corroborating and extending to CUD− our prior results in CUD+ (Goldstein et al., 2008). Post-hoc between-group analyses further showed that CUD− exhibited a significantly blunted P300 response to the 45¢ (highest reward task) condition [t(56)=2.21, p=0.031] and a similar trend in the 1¢ condition [t(56)=1.77, p=0.08] when compared to the other two study groups combined. We combined the CUD+ and controls as these groups did not differ in any of the monetary conditions [t(42)>−1.6, p>0.1]. Thus, consistent with our a priori hypothesis, the P300 amplitudes were lowest in the CUD− as directly compared to both CUD+ and healthy controls.
3.2. Behavioral results (Table 2)
3.2.1. Accuracy
The 3 × 3 mixed ANOVA revealed a group main effect [Controls=CUD+>CUD−: F(2,55)=5.9, p=0.005], such that CUD− were significantly less accurate than both controls and CUD+. Neither the main effect of money [F(2,54)=0.7, p=0.50] nor the money by group interaction [F(4,110)=0.7, p=0.59] reached significance. Mann-Whitney U test showed that the group main effect was driven by the lowest accuracy in the CUD− compared to CUD+ in the 0¢ condition [Z=−2.3, p=0.02; with the same trend observed during the 1¢ condition, Z=−1.9, p<0.1; given this preponderance of group differences in accuracy in the 0¢ and 1¢ conditions, and not in the 45¢ condition, lack of group differences in task earnings was not unexpected (Controls: $48.50 ± 1.33; CUD+: $47.31 ± 3.02; CUD−: $48.71 ± 1.07; F(2,54)=2.52, p=0.09)].
3.2.2. Reaction time
The 3 × 3 mixed ANOVA did not reveal a significant group main effect [F(2,55)=0.7, p=0.50], but did reveal a significant money main effect such that responses were fastest for the highest monetary condition across all study subjects [45¢<0¢: F(2,54)=4.9, p=0.01]. The money by group interaction was not significant [F(4,110)=0.6, p=0.65].
3.3. P300, behavior and drug use correlations
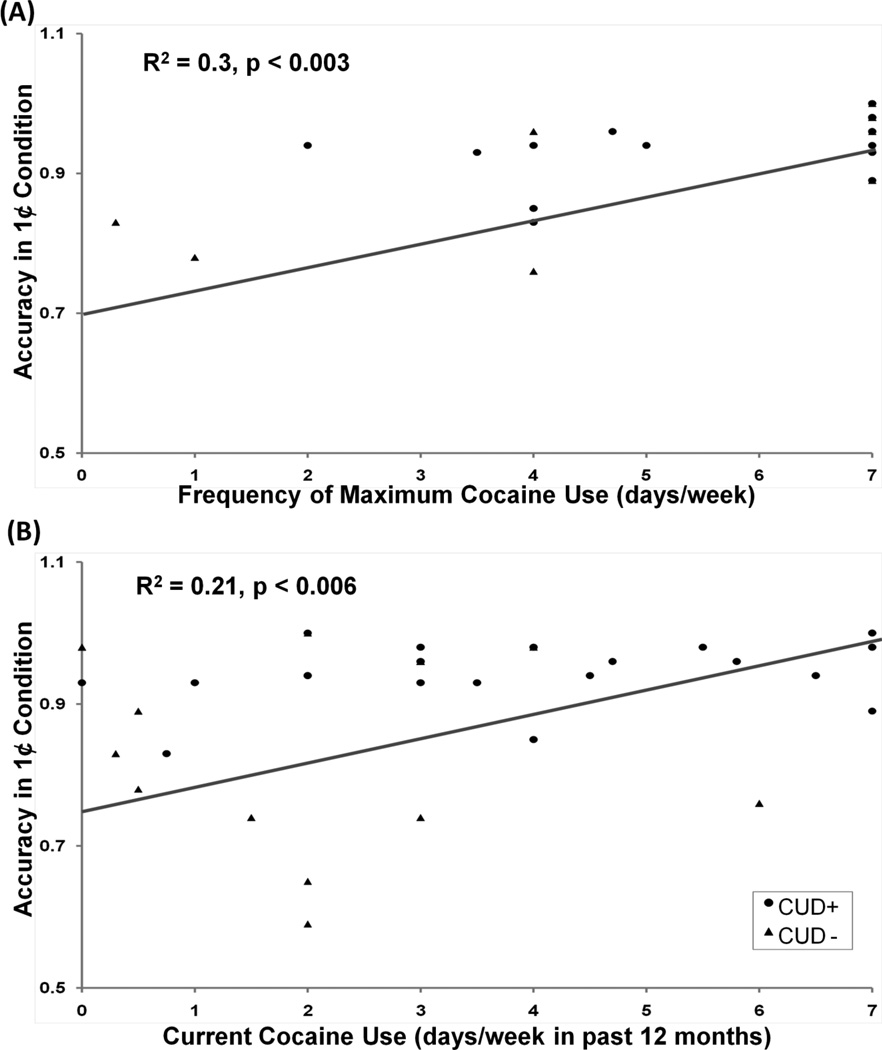
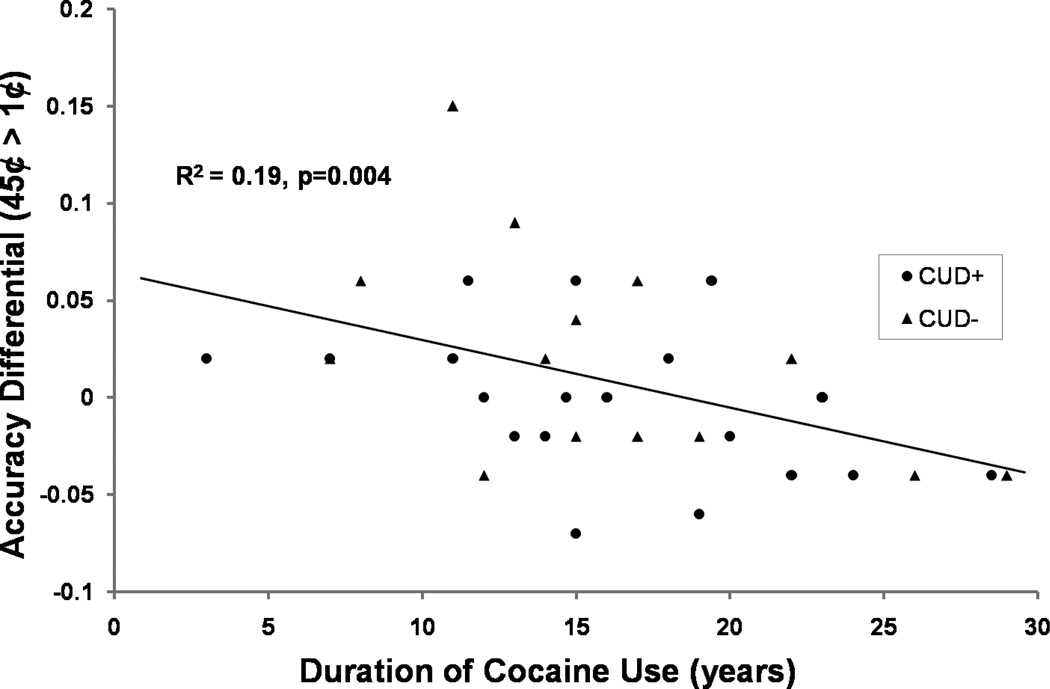
Across all CUD, there was a significant positive correlation between frequency of maximum cocaine use (days per week during the period of maximum cocaine use) with task accuracy during the 1¢ condition [r=0.52, p=0.002] (Figure 4A), with similar trends in the other two monetary conditions [r>0.35, p<0.05], as driven by the CUD+ group [r>0.53, p<0.05; in CUD− r<0.40, p>0.1). A similar correlation was observed between frequency of recent cocaine use (days per week in past 12 months) with task accuracy during the 1¢ condition [r=0.45, p=0.006] (Figure 4B), again driven by CUD+ [r=0.45, p=0.04; in CUD− r=0.12, p>0.1]. In contrast, across all CUD, duration of cocaine use (years) was negatively correlated with a task accuracy differential (45¢>1¢: r=−0.50, p=0.002; driven by CUD+: r=−0.44, p=0.05), such that the shorter the cocaine use, the better the accuracy response to high vs. low money (Figure 5). Moreover, in CUD+ only, a significant positive correlation was observed between frequency of maximum cocaine use (days per week during the period of maximum cocaine use) and P300 amplitude in response to 45¢ condition [r=0.49, p=0.028]. Taken together, these correlations suggest that the more frequent the drug use, the better the task accuracy in the CUD+ and the higher the P300 amplitudes. In contrast, chronic drug use was associated with lower behavioral accuracy to money across all CUD.
Figure 4.
Correlation between frequency of cocaine use and task-related variables. (A) and (B) show correlation between task accuracy for the 1¢ conditions and frequency (days per week) of maximum cocaine use and cocaine use in past 12 months in CUD (CUD+: ●; CUD−: ▲), respectively.
Figure 5.
Correlation between accuracy differential (45¢ > 1¢) and duration of cocaine use (years) in CUD (CUD+: ●; CUD−: ▲).
4. Discussion
In the current study we tested for the impact of recency of drug use/abstinence in cocaine addicted individuals on reward processing as measured with the P300, an ERP component reliably modulated by reward magnitude in healthy individuals (Hajcak et al., 2005; Sato et al., 2005; Wu and Zhou, 2009). For this purpose, we used monetary reward and compared its impact on the P300 between three subject groups: healthy controls, cocaine addicted individuals who tested positive (more frequent current users) and those who tested negative (less frequent and abstinent current users; reported here for the first time) for cocaine in urine. Earlier ERP components (P200 and N200) were used in exploratory analyses.
Consistent with our a priori hypothesis, severity of impairment (in P300 amplitudes and task accuracy) was most pronounced in the individuals with the least frequent recent cocaine use and a relatively longer abstinence compared to those with most frequent drug use and healthy controls. These results cannot be attributed to the effects of withdrawal given that the CUD subgroups did not differ in these symptoms as measured by the well standardized and widely used cocaine selective severity assessment scale (Kampman et al., 1998) (Table 1). These blunted P300 amplitudes, especially in the highest monetary condition, are consistent with previous studies where short-term abstinent CUD showed decreased P300 amplitudes in response to auditory oddball paradigms even when self-reported signs of withdrawal were minimal (Biggins et al., 1997; Kouri et al., 1996). The current study for the first time extends these results of general deficits in information processing (overall reduced P300 amplitude) to sensitivity to monetary reward (lack of P300 amplitude difference between reward and non-reward conditions), providing important evidence for a deficient response to a socially acquired and abstract reward despite its strong motivational and arousal value (i.e., association with drug procurement). Because P300 amplitude is a highly heritable phenotype (Almasy et al., 1999; Katsanis et al., 1997), results in the CUD− may also be driven by vulnerability factors, including familial history of drug use (Begleiter et al., 1984; Polich et al., 1994) or genetic predispositions (Hill et al., 1998; Porjesz et al., 2005), as remains to be separately ascertained.
Correlations between drug use variables, task behavior and P300 amplitude revealed that more frequent cocaine use is related to better task accuracy and higher P300 amplitude. Taken together with the results showing the most severe impairment in the CUD−, the correlations are consistent with cocaine’s neurocognitive enhancing effects (Higgins et al., 1990), providing support for the self-medication hypothesis, where drug abusers use their preferred drug to alleviate negative symptoms such as anhedonia, boredom susceptibility, low self-esteem (Khantzian, 1985), including avoidance of withdrawal symptoms (Duncan, 1974), and to mitigate underlying cognitive deficits (Woicik et al., 2009). The negative correlation between lifetime duration of cocaine use and sensitivity to monetary reward (task accuracy differential), however, provides a reminder that acute drug self-administration has a detrimental long-term effect, calling for the development of alternative interventions to ameliorate the underlying cognitive and emotional dysfunction in addiction. Additional explanations of results can include drug use expectation (Hogarth et al., 2007) and hypersensitivity to reward (Bechara et al., 2002), biased towards any monetary amount, as indicative of impaired processing of gradients of a reinforcer value in cocaine addiction (Goldstein et al., 2007b).
The relationship between cocaine use and task accuracy within the 1¢ condition (Figure 4) was unexpected and remains to be replicated and further explored in future studies. It also calls for some speculation. Compared to the 0¢ and 45¢ values, a physical coin with a 1¢ value physically exists in our environment. For CUD this may be the only true reward condition. Alternatively, CUD may perceive 1¢ as a negative reinforcer (a relative loss compared to the possibility of a 45¢ gain). Although lack of ERP (P200, N200 and P300) amplitude differences between the 1¢ and 45¢ conditions argues against these possibilities, a future study may address this issue by analyzing ERPs in response to feedback stimuli, especially the medial-frontal negativity (MFN) component, found to be sensitive to feedback indicating incorrectness of a response or monetary loss (Nieuwenhuis et al., 2004).
Both the P200 and N200 showed monetary modulation across all study subjects; the N200 further showed group effects. The P200 has been implicated in heightened attention to relevant cues (Carretie et al., 2001a; Carretie et al., 2001b; Naatanen and Michie, 1979) including reward-related stimuli (Franken et al., 2010; Martin and Potts, 2004). Therefore, lack of group main effect in the P200 amplitude shows that the overall task-related attention did not differ significantly between the study groups and that the P300 deficits in CUD− may specifically be related to deficits in reward-related information processing. Following earlier speculations that the P200 represents a necessary (although not sufficient) step before a P300 can be elicited (Garcia-Larrea et al., 1992), results suggest that early (as compared to more sustained) processing of money (and potentially of other motivational stimuli) may not be impacted in CUD. The N200 revealed significantly higher negativity for the non-reward (0¢) as compared to both reward conditions (1¢ and 45¢), in addition showing lowest amplitudes in the CUD+ (as compared to controls). Given earlier reports linking the N200 with discrimination of emotionally negative stimuli (Campanella et al., 2002; Liddell et al., 2004; Mayer and Merckelbach, 1999), these results may be driven by negative arousal, most pronounced during the non-reward task condition and in the more frequent current cocaine users. An alternative explanation invokes role of the N200 in indexing stimulus novelty arising from deviation from a predominant stimulus category (Daffner et al., 2000), with non-reward reflecting a deviation from the other reward trials. In such a case, the attenuated N200 response in CUD+ should have reflected higher response uncertainty and false-alarm rates (Falkenstein et al., 1999). However, there were no differences between CUD+ and controls in task accuracy. Nevertheless, these intriguing results warrant a follow-up study using tailored tasks to highlight these differences between CUD subgroups and controls.
We recognize the following limitations in the present report: (1) the blocked nature of the experimental design may have introduced habituation effects; (2) increasing sample size especially in the CUD− subgroup would allow examination of generalizability of results to longer abstinence periods; and (3) to more reliably test the self-medication hypothesis, the cognitive and emotional deficits putatively medicated by cocaine remain to be measured prior to initiation of cocaine use (i.e., in a longitudinal design). Future directions are therefore to (1) compare current results with longitudinal or protracted abstinence studies using test-retest within-subject design; (2) compare more disparate reward conditions (e.g., $2 vs. $1 vs. 10¢) and also add monetary loss (Yeung and Sanfey, 2004); (3) employ other analyses (e.g., LORETA) to refine the location of the neuroanatomical generators that are sensitive to reward salience; and (4) investigate the possibility of a reversal (or amelioration) of the reported deficits in the CUD− by administering a dopamine agonist (e.g., methylphenidate) or other (e.g., cognitive-behavioral) interventions.
In summary, the current results for the first time demonstrate that the more severe impairment in reward sensitivity characterizes CUD with less recent cocaine use/longer short-term abstinence. Taken together with the positive correlations with frequency of cocaine use, these results suggest that CUD may be acutely using cocaine to temporarily normalize underlying cognitive and emotional disruptions, albeit at the expense of longer-term detrimental impact on sensitivity to non-drug reward. These results emphasize the importance of developing treatment modalities, including pharmacological interventions, which would target improvements in neuropsychological function without reducing sensitivity to non-drug reward.
Supplementary Material
Acknowledgement
This work was supported by a grant from the National Institute on Drug Abuse [1R01DA023579 to RZG].
Notice: This manuscript has been authored by Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CHI-886 with the U.S. Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, a world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the United States Government purposes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almasy L, Porjesz B, Blangero J, Chorlian DB, O'Connor SJ, Kuperman S, Rohrbaugh J, Bauer LO, Reich T, Polich J, Begleiter H. Heritability of event-related brain potentials in families with a history of alcoholism. Am J Med Genet. 1999;88:383–390. [PubMed] [Google Scholar]
- Anokhin AP, Vedeniapin AB, Sirevaag EJ, Bauer LO, O'Connor SJ, Kuperman S, Porjesz B, Reich T, Begleiter H, Polich J, Rohrbaugh JW. The P300 brain potential is reduced in smokers. Psychopharmacology (Berl) 2000;149:409–413. doi: 10.1007/s002130000387. [DOI] [PubMed] [Google Scholar]
- Bauer LO. CNS recovery from cocaine, cocaine and alcohol, or opioid dependence: a P300 study. Clin Neurophysiol. 2001;112:1508–1515. doi: 10.1016/s1388-2457(01)00583-1. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Biggins CA, MacKay S, Clark W, Fein G. Event-related potential evidence for frontal cortex effects of chronic cocaine dependence. Biol Psychiatry. 1997;42:472–485. doi: 10.1016/S0006-3223(96)00425-8. [DOI] [PubMed] [Google Scholar]
- Bruin KJ, Wijers AA. Inhibition, response mode, and stimulus probability: a comparative event-related potential study. Clin Neurophysiol. 2002;113:1172–1182. doi: 10.1016/s1388-2457(02)00141-4. [DOI] [PubMed] [Google Scholar]
- Campanella S, Gaspard C, Debatisse D, Bruyer R, Crommelinck M, Guerit JM. Discrimination of emotional facial expressions in a visual oddball task: an ERP study. Biol Psychol. 2002;59:171–186. doi: 10.1016/s0301-0511(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Carretie L, Martin-Loeches M, Hinojosa JA, Mercado F. Emotion and attention interaction studied through event-related potentials. J Cogn Neurosci. 2001a;13:1109–1128. doi: 10.1162/089892901753294400. [DOI] [PubMed] [Google Scholar]
- Carretie L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the 'negativity bias', studied through event-related potentials. Int J Psychophysiol. 2001b;41:75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Chen AC, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, Chorlian DB, Stimus AT, Begleiter H. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol Clin Exp Res. 2007;31:156–165. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Curry SH, Cooper R, McCallum WC, Pocock PV, Papakostopolous D, Skidmore S, Newton P. The principle components of auditory target detection. In: Gaillar AWK, Ritter W, editors. Tutorials in event-related potentials research: endogenous components. New York: North-Holland Publishing; 1983. pp. 79–118. [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LF, Calvo V, Faust R, Holcomb PJ. An electrophysiological index of stimulus unfamiliarity. Psychophysiology. 2000;37:737–747. [PubMed] [Google Scholar]
- de Ruiter MB, Veltman DJ, Goudriaan AE, Oosterlaan J, Sjoerds Z, van den Brink W. Response perseveration and ventral prefrontal sensitivity to reward and punishment in male problem gamblers and smokers. Neuropsychopharmacology. 2009;34:1027–1038. doi: 10.1038/npp.2008.175. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: an open source program for advanced statistical analysis of event-related potential data. J Neurosci Methods. 2010;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J, Beal DJ, Berg P. Optimizing principal components analysis of event-related potentials: matrix type, factor loading weighting, extraction, and rotations. Clin Neurophysiol. 2005;116:1808–1825. doi: 10.1016/j.clinph.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Duncan DF. Drug-Abuse as a Coping Mechanism. Am J Psychiat. 1974;131:724–724. doi: 10.1176/ajp.131.6.724. [DOI] [PubMed] [Google Scholar]
- Dunning JP, Parvaz MA, Hajcak G, Maloney T, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users - an ERP study. Eur J Neurosci. 2011;33:1716–1723. doi: 10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol (Amst) 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Fein G, Chang M. Visual P300s in long-term abstinent chronic alcoholics. Alcohol Clin Exp Res. 2006;30:2000–2007. doi: 10.1111/j.1530-0277.2006.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Williams J. Structured Clinical Interview for DSM-IV Axis I disorders - Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biol Psychol. 2009;81:1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Franken IH, Van den Berg I, Van Strien JW. Individual differences in alcohol drinking frequency are associated with electrophysiological responses to unexpected nonrewards. Alcohol Clin Exp Res. 2010;34:702–707. doi: 10.1111/j.1530-0277.2009.01139.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Lukaszewicz AC, Mauguiere F. Revisiting the oddball paradigm. Non-target vs neutral stimuli and the evaluation of ERP attentional effects. Neuropsychologia. 1992;30:723–741. doi: 10.1016/0028-3932(92)90042-k. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007a;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Cottone LA, Jia Z, Maloney T, Volkow ND, Squires NK. The effect of graded monetary reward on cognitive event-related potentials and behavior in young healthy adults. Int J Psychophysiol. 2006;62:272–279. doi: 10.1016/j.ijpsycho.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Parvaz MA, Maloney T, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND. Compromised sensitivity to monetary reward in current cocaine users: an ERP study. Psychophysiology. 2008;45:705–713. doi: 10.1111/j.1469-8986.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, Volkow ND. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug Alcohol Depend. 2007b;87:233–240. doi: 10.1016/j.drugalcdep.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding DC, Burroughs S, Boutros NN. Attentional deficits in cocaine-dependent patients: converging behavioral and electrophysiological evidence. Psychiatry Res. 2008;160:145–154. doi: 10.1016/j.psychres.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology. 2005;42:161–170. doi: 10.1111/j.1469-8986.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR, Lynn M, Capeless MA, Fenwick JW. Effects of intranasal cocaine on human learning, performance and physiology. Psychopharmacology (Berl) 1990;102:451–458. doi: 10.1007/BF02247124. [DOI] [PubMed] [Google Scholar]
- Hill SY, Locke J, Zezza N, Kaplan B, Neiswanger K, Steinhauer SR, Wipprecht G, Xu J. Genetic association between reduced P300 amplitude and the DRD2 dopamine receptor A1 allele in children at high risk for alcoholism. Biol Psychiatry. 1998;43:40–51. doi: 10.1016/s0006-3223(97)00203-5. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Dickinson A, Wright A, Kouvaraki M, Duka T. The role of drug expectancy in the control of human drug seeking. J Exp Psychol Anim Behav Process. 2007;33:484–496. doi: 10.1037/0097-7403.33.4.484. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, Krigolson OE. The feedback correct-related positivity: sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45:688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D'Angelo L, Epperson LE. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK, Carlson SR. P300 event-related potential heritability in monozygotic and dizygotic twins. Psychophysiology. 1997;34:47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Lukas SE, Mendelson JH. P300 assessment of opiate and cocaine users: effects of detoxification and buprenorphine treatment. Biol Psychiatry. 1996;40:617–628. doi: 10.1016/0006-3223(95)00468-8. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kaufman DA, Perlstein WM. Conflict adaptation and cognitive control adjustments following traumatic brain injury. J Int Neuropsychol Soc. 2009;15:927–937. doi: 10.1017/S1355617709990701. [DOI] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Williams LM, Rathjen J, Shevrin H, Gordon E. A temporal dissociation of subliminal versus supraliminal fear perception: an event-related potential study. J Cogn Neurosci. 2004;16:479–486. doi: 10.1162/089892904322926809. [DOI] [PubMed] [Google Scholar]
- Martin LE, Potts GF. Reward sensitivity in impulsivity. Neuroreport. 2004;15:1519–1522. doi: 10.1097/01.wnr.0000132920.12990.b9. [DOI] [PubMed] [Google Scholar]
- Mayer B, Merckelbach H. Unconscious processes, subliminal stimulation, and anxiety. Clin Psychol Rev. 1999;19:571–590. doi: 10.1016/s0272-7358(98)00060-9. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Michie PT. Early selective-attention effects on the evoked potential: a critical review and reinterpretation. Biol Psychol. 1979;8:81–136. doi: 10.1016/0301-0511(79)90053-x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Holroyd CB, Mol N, Coles MG. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neurosci Biobehav Rev. 2004;28:441–448. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- Papageorgiou CC, Liappas IA, Ventouras EM, Nikolaou CC, Kitsonas EN, Uzunoglu NK, Rabavilas AD. Long-term abstinence syndrome in heroin addicts: indices of P300 alterations associated with a short memory task. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1109–1115. doi: 10.1016/j.pnpbp.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Potts GF. An ERP index of task relevance evaluation of visual stimuli. Brain Cogn. 2004;56:5–13. doi: 10.1016/j.bandc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Potts GF, Dien J, Hartry-Speiser AL, McDougal LM, Tucker DM. Dense sensor array topography of the event-related potential to task-relevant auditory stimuli. Electroencephalogr Clin Neurophysiol. 1998;106:444–456. doi: 10.1016/s0013-4694(97)00160-0. [DOI] [PubMed] [Google Scholar]
- Potts GF, Martin LE, Burton P, Montague PR. When things are better or worse than expected: the medial frontal cortex and the allocation of processing resources. J Cogn Neurosci. 2006;18:1112–1119. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- Pritchard WS. Psychophysiology of P300. Psychol Bull. 1981;89:506–540. [PubMed] [Google Scholar]
- Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behav Ecol. 2008;19:690–693. [Google Scholar]
- Sato A, Yasuda A, Ohira H, Miyawaki K, Nishikawa M, Kumano H, Kuboki T. Effects of value and reward magnitude on feedback negativity and P300. Neuroreport. 2005;16:407–411. doi: 10.1097/00001756-200503150-00020. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- Stevens J. Applied multivariate statistics for the social sciences. Belmont, CA: 1992. [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- van Erp TGM, Saleh PA, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Cannon TD. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiat. 2002;159:1514–1520. doi: 10.1176/appi.ajp.159.9.1514. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, Volkow ND, Goldstein RZ. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34:1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhou X. The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Res. 2009;1286:114–122. doi: 10.1016/j.brainres.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Yang B, Yang S, Zhao L, Yin L, Liu X, An S. Event-related potentials in a Go/Nogo task of abnormal response inhibition in heroin addicts. Sci China C Life Sci. 2009;52:780–788. doi: 10.1007/s11427-009-0106-4. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. J Neurosci. 2004;24:6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.