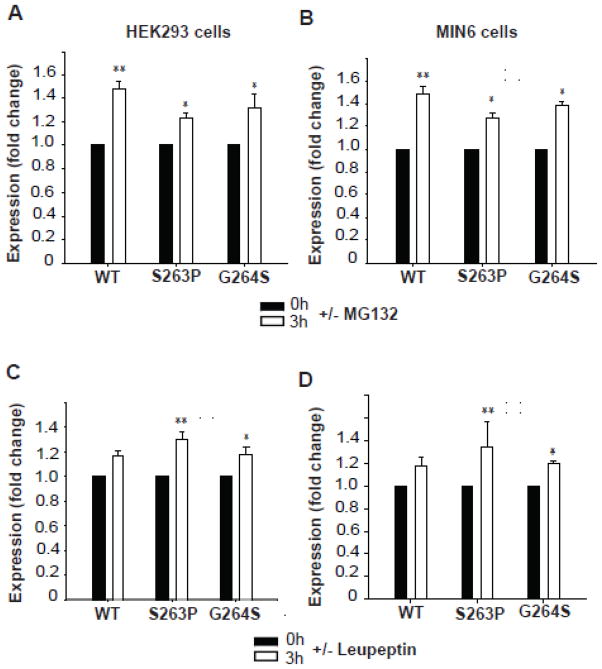
Fig. 5.
Effect of proteasomal and lysosomal inhibitors on the apparent cellular stability of WT and mutant hGK forms. The recovery of the ~53 kDa band of WT hGK and the S263P and G264S mutant forms in stably transfected HEK293 cells (A and C) and MIN6 cells (B and D) was followed in pulse-chase experiments. The cells were labeled with [35S]Met/[35S]Cys for 30 min and chased at 0 h (black columns) and 3 h (white columns), in the presence and absence of proteasomal inhibitor (A and B) and lysosomal inhibitor (C and D). Cell lysis was followed by immunoprecipitation (anti-V5 Ab) of total detergent soluble GK. Samples were denatured and analyzed by SDS/PAGE (4–12 %), autoradiography and densitometric analysis of the radioactive bands. Each column represents the mean ± SD of triplicates examined on three independent days (n = 9). The fold change after 3 h refers to the difference in the average recovery at t = 3 h relative to the average recovery at t = 0 h (=1.0).