Fig. 1.
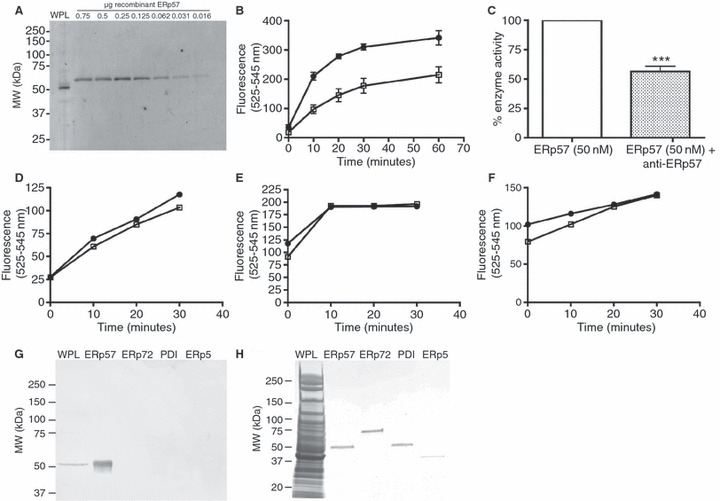
The generation of anti-ERp57 activity blocking antibodies which selectively inhibit ERp57. Human platelets (4 × 106 cells) and recombinant protein (0.75–0.016 μg) were separated by SDS-PAGE and immunoblotted to detect ERp57 (anti-ERp57, 1:1000 dilution in 2% (w/v) skimmed milk powder/TBS-T) and anti-sheep IgG HRP-conjugated antibody (1:4000 dilution in 2% (w/v) skimmed milk powder/TBS-T) then developed using by chemiluminescence (A). The ability of anti-ERp57 (37.5 μg mL−1) to inhibit the activity of recombinant ERp57 (50 nm) was measured by fluorescence substrate-based thiol isomerase assay (B). Anti-ERp57 was incubated with enzyme for 5 min prior to addition to 500 μL DI-E-GSSG substrate in the presence of 5 μm DTT (closed circles: enzyme alone, open squares: enzyme with anti-ERp57). Enzyme activity was calculated and % inhibition of ERp57 activity at 30 min demonstrated in graph (C). Cross reactivity of anti-ERp57 with recombinant (D) PDI, (E) ERp72, (F) ERp5 (all 50 nm) was measured by thiol isomerase assay and (G) immunoblotting (5 μg recombinant enzyme/lane). Immunoblots were stained with coomassie blue stain to reveal protein loading (H).
