Figure 2.
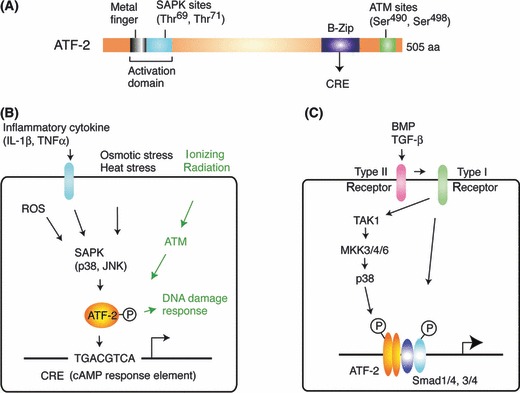
ATF-2 is a nuclear target of p38/Jun N-terminal protein kinase (JNK). (A) Domain structure of ATF-2. ATF-2 contains a B-ZIP-type DNA-binding domain and a transactivation domain, which consists of a metal finger structure and SAPK phosphorylation sites. (B) ATF-2 is activated by SAPKs in response to various stresses, including inflammatory cytokines, environmental stresses, and DNA damage. These stresses induce the phosphorylation of ATF-2 via SAPKs, which then activates the transcription of target genes. DNA double-strand breaks induce the phosphorylation of ATF-2 via ATM, which then participates in DNA repair. (C) Role of ATF-2 in the bone morphogenetic protein (BMP)/TGF-β signaling pathway. ATF-2 is phosphorylated via the TAK1-p38 pathway in response to BMP/TGF-β, although BMP/TGF-β induces the phosphorylation of Smad1 or Smad3, which leads to the nuclear entry of the Smad1/4 or Smad3/4 complex. In the nucleus, ATF-2 interacts with the Smad complex and synergistically activates transcription.
