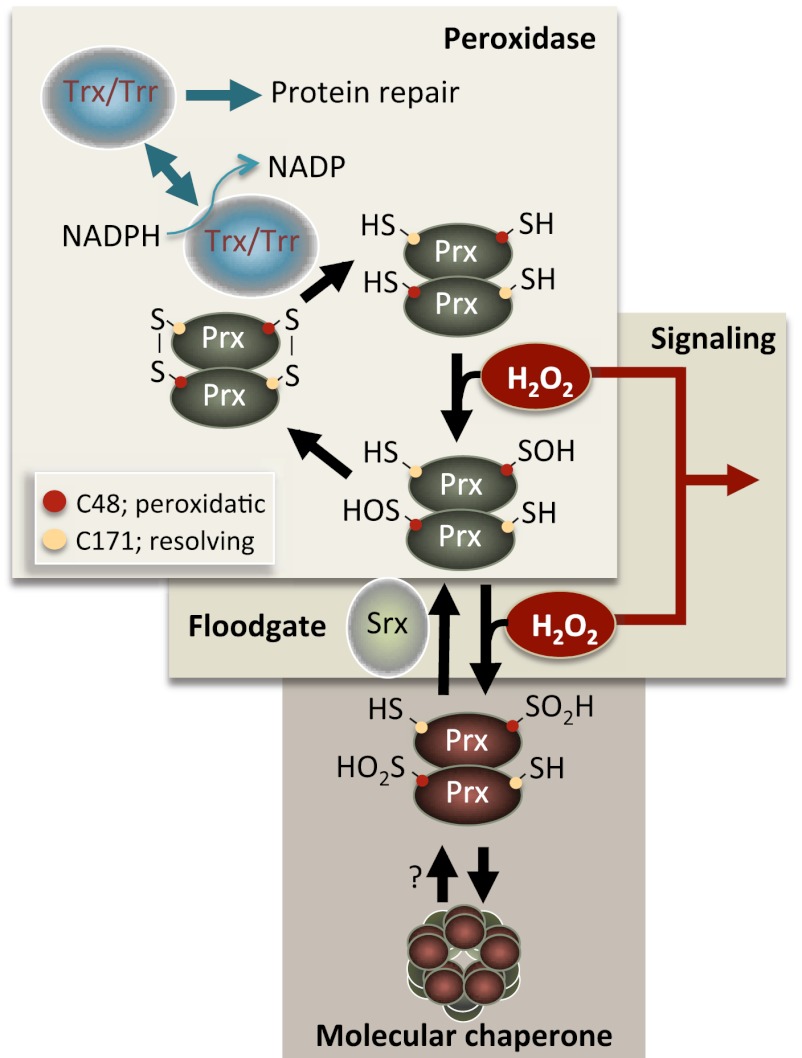
Figure 1.
Overview of catalysis in typical 2-Cys Prx. Hydrogen peroxide (H2O2) is reduced by the peroxidatic Cys that is oxidized to a sulfenic acid intermediate (C48-SOH in yeast). The nascent R-SOH then condenses with the resolving Cys (C171-SH in yeast) of the other Prx molecule in the Prx dimer to produce a disulfide bond. This bond is subsequently reduced by Trx, thus completing the catalytic cycle. A proportion of Cys-SOH molecules can be further oxidized (hyperoxidation) to the sulfinic acid form (Cys-SOOH) in each catalytic cycle, which inactivates peroxidase activity (Wood et al. 2003b). Srxs reduce the sulfinylated forms of 2-Cys Prxs (Biteau et al. 2003; Woo et al. 2005), which allows Prx to re-enter the catalytic, peroxidatic cycle. The hyperoxidized form of Prx forms oligomers (Hall et al. 2011; Saccoccia et al. 2012) that act as molecular chaperones (i.e., inhibit protein aggregation in vitro) (Jang et al. 2004). The boxes denote three principal Prx activities that may affect genome stability and life span: (1) peroxidase activity reducing the levels of harmful peroxides, (2) hyperoxidation by the “floodgate reaction” allowing peroxide levels to reach local thresholds as a second messenger in signal transduction, and (3) hyperoxidation leading to Prx oligomer formation and molecular chaperone activity. (Trr) Trx reductase.