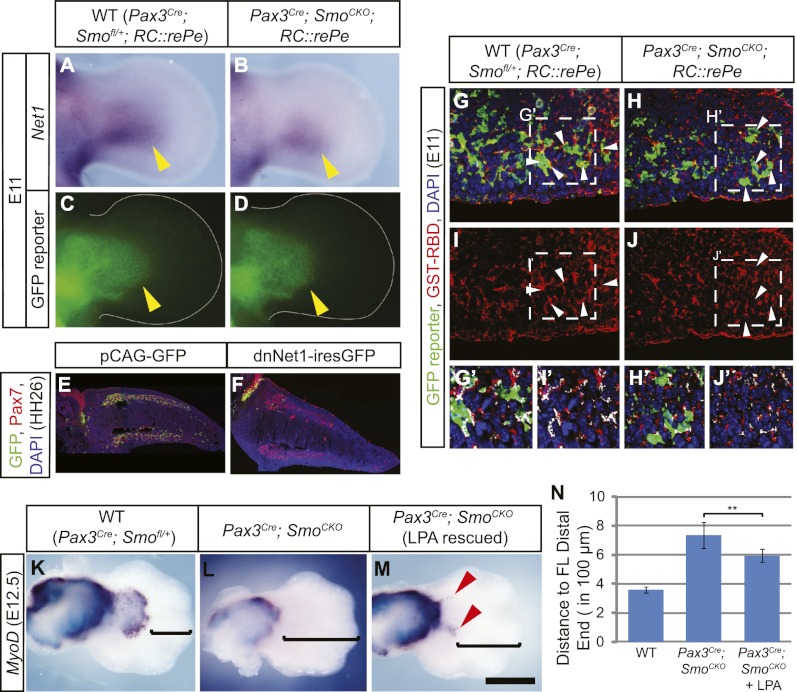
Figure 7.
Net1 acts downstream from Hh signaling to promote cell migration. (A,B) Net1 in situ hybridization showed that Net1 expression was down-regulated in the Pax3Cre; SmoCKO; RC∷rePe mutant forelimb (B, yellow arrowhead) at E11 when compared with the wild-type (WT) limb bud (A). (C,D) This down-regulation was not due to a loss of muscle progenitors in the limb, marked by the Pax3Cre-responsive GFP reporter. (E,F) In the chick system, when the lateral somites at the hindlimb level were electroporated with pCAG-GFP at HH18, GFP-positive cells (green) were found throughout the Pax7-positive dorsal and ventral muscle masses (red) at HH26 (shown in E). (F) However, when two dominant-negative forms of Net1 were coelectroporated into the lateral somites, cells failed to migrate into the limb, demonstrating that functional Net1 activity is required for muscle cell migration. (G–J) Active RhoA, as detected by GST-RBD, was reduced in the Pax3Cre; SmoCKO; RC∷rePe mutant forelimb ventral muscle cells, marked by the Cre-responsive GFP reporter, at E11 (white arrowheads). (G′–J′) Blow-ups of the squares in G–J show that there are less overlapping green and red signals (white patches) in the mutant myogenic cells. (K–M) The loss of distal forelimb muscles in the Pax3Cre; SmoCKO mutants was partially rescued (red arrowheads) by serial injection of the RhoA activator LPA. (N) This rescue can be quantified as the distance from the distal-most MyoD staining to the tip of the hand plate (as indicated by the brackets in K–M). Histograms are expressed as means and standard error of the mean (SEM) (n = 6 for each genotype). (**) P < 0.01. Bar in M: A–D, 315 μm; E,F, 350 μm; G–J, 60 μm; G′–J′, 45 μm; K–M, 500 μm.