Abstract
A series of 3,5-bis (4-hydroxyphenyl) isoxazoles bearing a styryl/alkyl vinyl group at the 4-position were prepared and evaluated as ligands for the estrogen receptor-alpha (ERα). The target compounds were prepared using the Suzuki reaction to couple an iodo-isoxazole intermediate with a series of styryl/alkenyl boronic acids, followed by O-demethylation. The products were evaluated for their estrogen receptor-α ligand binding domain (ERα-LBD) binding affinity using a competitive binding assay. The 4-(4-hydroxystyryl) derivative 4h displays binding properties similar to those of the previously described pyrazole class of ER ligands, indicating that the ERα-LBD tolerates the presence of the added vinyl group at the 4-position of the isoxazole ring.
Keywords: Estrogen receptors, isoxazoles, synthesis, Suzuki coupling reaction, receptor binding, conformations
1. Introduction
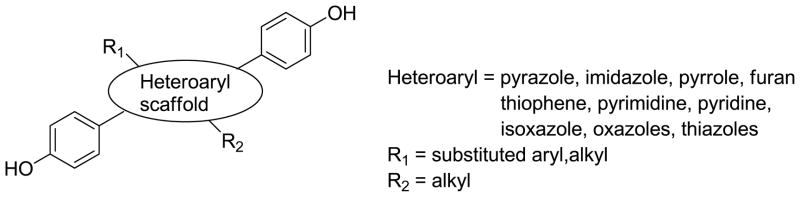
The estrogen receptor displays the capacity to bind a wide variety of nonsteroidal ligands and to generate diverse biological responses as a consequence of that binding interaction.[1] A number of investigators have undertaken efforts to systematically prepare and evaluate non-steroidal ligand cores. The strategy, exemplified by the work of Katzenellenbogen and others, typically involved the preparation of a bis(4-hydroxyphenyl) heteroarene to mimic the estradiol skeleton and the incorporation of additional functional groups (R1/R2) that would enhance ER affinity and influence efficacy.[2–18][Figure 1] Relative binding affinities (RBA) for these derivatives ranged from 0.01–140% (compared to estradiol-100%), depending both upon the nature of the heteroaryl group and the R1/R2 substituents.
Figure 1.
Nonsteroidal ER ligands based on bis(4-hydroxyphenyl) heteroarene scaffold and incorporating additional R1 and R2 substituents.
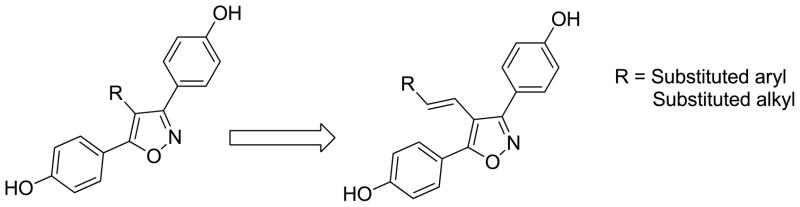
As part of our program to identify new structural motifs that elicit the desired ER response, we also focused on the preparation of new di- and tri-aryl heteroarenes. Although 3,5-diaryl isoxazoles are electronically similar to the 3,5-diaryl pyrazoles, isoxazole analogs had not been as extensively evaluated for estrogen ligand development as the corresponding pyrazoles.[10,18]. We noted that incorporation of a CH2-, C=O or CH2O-linker between the isoxazole ring and the aryl group gave products with significant ER affinity, however, none of the derivatives had employed a vinyl group to link the isoxazole core with the terminal (substituted phenyl) moiety. We hypothesized that an appropriate styryl/alkylvinyl group would yield derivatives that retain signicant affinity for the ERα-LBD. [Figure 2] In addition, the synthetic approach should contain elements that would make it amenable to combinatorial chemistry, thereby permitting rapid exploitation of the chemical space. This paper describes the synthesis and receptor binding results with this series of new 4-(styryl/alkenyl)-isoxazole derivatives.
Figure 2.
Transformation of 4-substituted-3,5-bis(4-hydroxyphenyl) isoxazoles to corresponding 4-styryl/alkylvinyl-3,5-bis(4-hydroxyphenyl) isoxazoles.
2. Results and Discussion
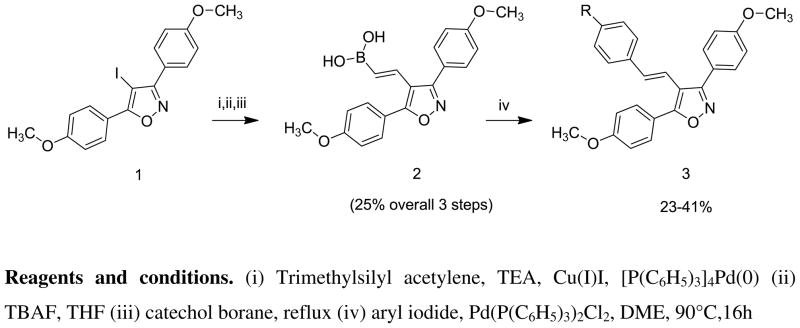
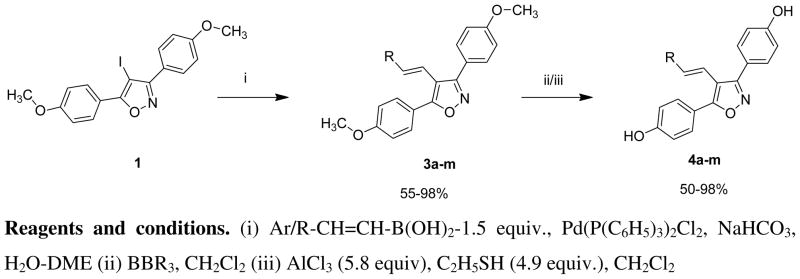
Our synthetic objective involved the development of a direct, reliable synthetic route to the preparation of the target compounds. We examined two approaches, based upon a 4-iodo-3,5-bis(4-methoxyphenyl)-isoxazole intermediate, either of which could be expanded to combinatorial/parallel synthesis. In the first approach [Scheme 1] we converted the 4-iodo intermediate 1 [18] to the 4-vinyl boronic acid derivative 2 using the Sonogashira reaction,[19,20] followed by catechol hydroboration and hydrolysis. Subsequent Suzuki coupling with substituted aryl halides gave 4-(substituted styryl)-3,5-bis(4-methoxyphenyl) isoxazoles 3. The Sonogashira reaction, however, proceeded in low yield (~30%) and was accompanied by side products that required careful separation. Although the yield for the hydroboration step was satisfactory (77%), the yields for the subsequent Suzuki reactions to give the 4-(substituted styryl)-3,5-bis(4-methoxyphenyl) isoxazoles 3 were low (20–40%). Therefore we developed the second approach, (Scheme 2) in which we coupled the 4-iodo intermediate 1 with a series of commercially available substituted styryl and alkylvinyl boronic acids. In this case, the yields for the Suzuki reaction were very good (56–98%). Subsequent O-demethylation of the intermediates 3a–m, using boron tribromide or aluminum trichloride-ethyl mercaptan gave the initial series of target compounds 4a–m in good yields (53–98%).
Scheme 1.
Synthesis of 4-styryl-3,5-bis(4-methoxyphenyl) isoxazoles 3 via the 4-vinylboronic acid intermediate.
Scheme 2.
Synthesis of 4-styryl-3,5-bis(4-hydroxyphenyl) isoxazoles 4a–4m via the 4-iodo intermediate and commercially available styryl/alkylvinyl boronic acids.
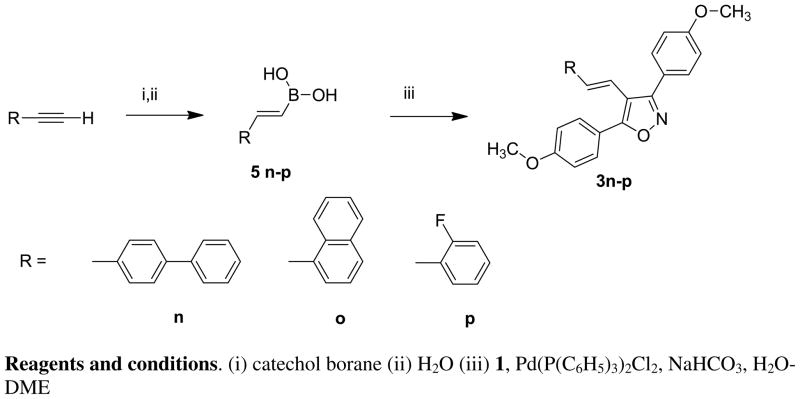
Although the second approach was synthetically more successful, it is limited by the availability of coupling partners, i.e., arylvinyl boronic acids are not as readily available as aryl halides. As a basis for chemical library design, we demonstrated that other arylvinyl boronic acids could be readily accessed for use in this synthetic strategy. Conversion of aryl halides to the corresponding aryl acetylenes using the Sonogashira reaction has been well described. For our study, we used three such alkynes and converted them to the corresponding aryl vinyl boronic acids [21] and subsequently to the isoxazole derivatives. [Scheme 3] The yields for the catechol hydroboration, hydrolysis (43–61%) and coupling steps for the representative compounds 5n–p [22–24] were very satisfactory (43–61%). Conversion to the isoxazole derivatives 3n–p also proceeded in good yields (73–93%).
Scheme 3.
Synthesis of novel arylvinyl boronic acids 5n–p and conversion to 4-(substituted styryl)-3,5-bis(4-methoxyphenyl) isoxazole derivatives 3n–p.
The initial series of 4-(substituted styryl/alkenyl)-3,5-bis(4-hydroxyphenyl)isoxazoles 4a–4m were evaluated as ligands for ERα-LBD using a competitive binding assay [25,26]. The relative binding affinity (RBA) values for the new compounds are shown in Table 1, where estradiol has a value of 100%. All of the compounds demonstrated significant affinity for the ERα-LBD, with values ranging from <1–16% that of estradiol. The highest affinity was observed for the 4-hydroxystyryl derivative 4h (16.4 %), with the 3-hydroxy isomer 4i (8.7%) slightly lower. Substitution of the ring at the 4-position with alkyl or halogenated groups (4c–g) reduced binding affinity (RBA <1%-5.3%) and as did replacement of the aromatic ring by alkyl groups (4j–m, RBA =1.3–4.3%). The Z-styryl isomer 4b appeared to be a slightly better ligand for ERα-LBD than the E-isomer 4a (8.7 vs 4%). Because substitution on the phenyl ring with groups other than hydroxyl appeared to reduce the binding affinity, we did not convert isoxazole derivatives 3n–3p to the corresponding bis(4-hydroxyphenyl) analogs 4n–4p for evaluation.
Table 1.
Relative Binding Affinities (RBA) of Isoxazole derivatives for the Estrogen Receptor-α Ligand Binding Domain (ERα-LBD).
| ||
|---|---|---|
| Compound | R = | RBA ±S.D |
| 4a | E-C6H5 | 3.5±1.0% |
| 4b | Z-C6H5 | 8.7±1.2 |
| 4c | 4-CH3-C6H5 | 0.6±0.2% |
| 4d | 4-F-C6H5 | 3.1±0.7% |
| 4e | 4-Cl-C6H5 | 0.8±0.2% |
| 4f | 4-CF3-C6H5 | 5.3±1.2 |
| 4g | 3-CF3-C6H5 | 1.5±0.7 |
| 4h | 4-HO-C6H5 | 16.4±3.8 |
| 4i | 3-HO-C6H5 | 8.7±1.5 |
| 4j | n-C7H15 | 1.8±1.2 |
| 4k | n-C8H17 | 2.5±0.7 |
| 4l | n-C9H19 | 1.3±0.6 |
| 4m | t-C4H9 | 4.3±3.2 |
RBA = 100 × [E]/[C] where [E] is the concentration of unlabeled estradiol necessary to reduce the specific binding of [3H]-estradiol to the ERα-LBD by 50% and [C] is the concentration of the competitive ligand necessary to reduce specific binding by 50%. The RBA of estradiol is 100% at 25 °C. Curves for ligand and estradiol had correlation coefficients >95%.
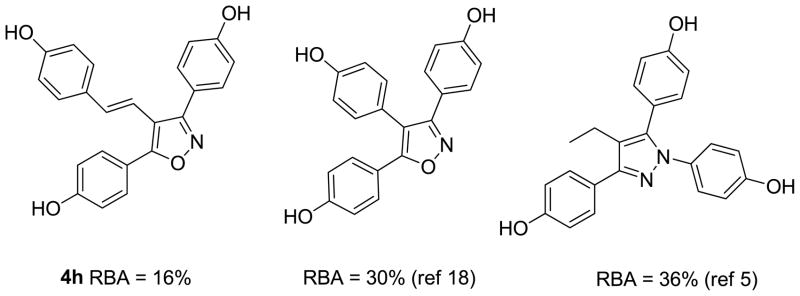
The binding properties of this series of tri-substituted isoxazoles compares favorably with previous studies on tris(4-hydroxyphenyl) five membered heterocycles. For example, the Chiron patent [18] reported a relative binding affinity (RBA) of 30% for 3,4,5- tris(4-hydroxyphenyl) isoxazole. In our case, introduction of the vinyl group between the isoxazole ring and the aryl group at the 4-position appears to be well tolerated in terms of ERα-LBD binding. The binding of the new compounds also compares favorably with the other heterocyclic analogs. Most of the tris(4-hydroxyphenyl) substituted pyrazoles reported by Katzenellenbogen also have RBA values in the 5–25% range. [3,5,8,10,11] Figure 3 presents the RBA value for 4h compared to two of the more potent isoxazole and pyrazole compounds.
Figure 3.
Comparison of compound 4h with similar tris(4-hydroxyphenyl) heteroarenes.
The RBA values for the 4- and 3-hydroxyphenyl derivatives 4h and 4i are also comparable to the highest values observed for the tri- and tetrasubstituted pyrrole ER ligands recently reported by Gust. [14,15] It should be noted that pyrazoles and furans which have higher reported RBA values than 4h may achieve the enhanced affinity through the additional fourth substituent. This is not possible with the isoxazole scaffold. By comparison, the substituted isoxazoles described in this study generally were better than the previously reported imidazoles, oxazoles and thiazole which had very low RBA (<1%) values for ER. [10]
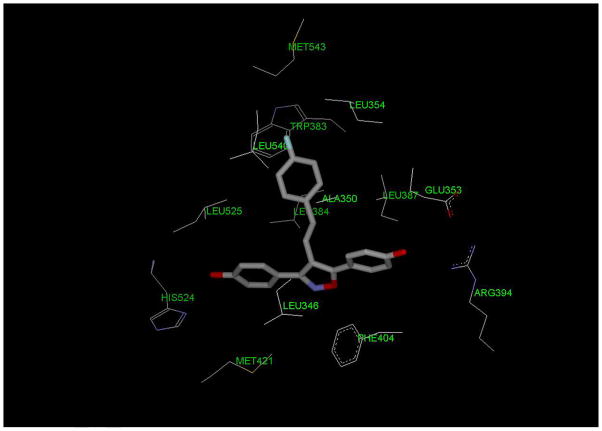
Based prior studies of the interaction between nonsteroidal ligands and ERα-LBD, we proposed a binding mode for the new ligands that provides a rationale for the structure-activity relationship. We illustrated this mode using compound 4d whose RBA value is less than that of the highest affinity agent 4h but somewhat more than the less active alkyl vinyl derivatives. [27] As with most nonsteroidal ligands of this type, the 3,5-bis(4- hydroxyphenyl)-isoxazole scaffold mimics the internal steroidal core of estradiol. The vinyl moiety at the 4-position experiences torsional strain due to the neighboring (4-fluorophenyl) groups and adopts a non-coplanar conformation that will reduce the steric strain. The more distal vinyl-aryl bond can also undergo rotation to achieve the most effective ligand-ERα-LBD contacts and further lower the energy of the complex. Our study (Figure 4) suggests that the terminal 4-fluoro-group interacts with residues Leu-540(helix-12) and Trp-383(helix5), as well as Thr-347 (helix-3), which is not see in this depiction. Hydrogen bonding by either the 4-hydroxyl (4h) or 3-hydroxyl (4i) group provides additional stabilization within this pocket. Hydrophobic substituents such as choro- (4e), methyl (4c) or trifluoromethyl (4f,4g) are tolerated but not favored. The alkyl vinl derivatives 4j–4m are also accommodated within this hydrophobic pocket, but provide no additional interactions that would strengthen binding. Other modeling studies have identified Thr-347 as the residue in the 11β-pocket most likely to establish hydrogen bonding with the distal phenolic hydroxyl.[4,5] This would appear to be the case as the two ligands with the highest RBA values were the 4- and 3-hydroxystyryl isoxazoles 4h and 4i. Substitution with non-hydrogen bonding or hydrophobic groups reduces the binding interaction, but conformation mobility may result in alternative binding modes that generate stable complexes. One interesting observation in this series was the difference between the 4-E- and 4-Z-styryl isoxazoles where the Z-isomer 4b had a slightly higher affinity than the E-isomer 4a (8.7% vs 4%). It is possible that the terminal phenyl group of the Z-derivative can access a slightly different and/or more favorable binding pocket than the E-counterpart.
Figure 4.
Docking study with isoxazole 4d and ERα-LBD showing interaction between 4-(4-fluorophenyl)-group and Leu-540 and Trp-383 residues.
3. Conclusions
In this study we developed an approach to the synthesis of 4-(substituted styryl)-3,5-bis(4-hydroxyphenyl) isoxazoles that is efficient and can be extended to combinatorial expansion. Although limited by the availabaility of the substituted styryl boronic acids, such reagents can be prepared from commercially available starting materials. The resultant products demonstrate significant binding affinity for the ERα-LBD that is comparable to previously described bis- and tris-(4-hydroxyphenyl) isoxazole, pyrazole and furan derivatives. The presence of the vinyl group at the 4-position is well tolerated and may permit the distal 3-/4-hydroxyphenyl moiety, present in compounds 4h and 4i, to adopt conformations that can access complementary residues, such as Thr-347, Trp-383, and Leu-540 in the 11β-binding pocket. The results provide the basis for future studies that evaluate the relationship between the 4-substituent and estrogenic potency. Studies in which the 4-styryl group is substituted with dialkylaminoethoxy groups are in progress as well.
Supplementary Material
Acknowledgments
The authors acknowledge support for this research through grants from the National Institutes of Health [PHS 1RO1 CA81049 (R.N.H.), PHS 1R01 CA 37799 and R21MH082252 (R.B.H.)], and the U.S. Army Breast Cancer Research Program [DAMD 17-99-1- 9333 and 17-00-1-00384 (R.N.H.)]. The substituted vinyl boronic acids were provided by Frontier Scientific for which the authors are particularly grateful.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gao H, Katzenellenbogen JA, Garg R, Hansch C. Chem Rev. 1999;99:723. doi: 10.1021/cr980018g. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh U, Ganessunker D, Sattigeri VJ, Carlson KE, Mortensen DS, Katzenellenbogen BS, Katzenellenbogen JA. Bio-org Med Chem Lett. 2003;11:629. doi: 10.1016/s0968-0896(02)00309-7. [DOI] [PubMed] [Google Scholar]
- 3.Mortensen DS, Rodriguez AL, Carlson KE, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. J Med Chem. 2001;44:3838. doi: 10.1021/jm010211u. [DOI] [PubMed] [Google Scholar]
- 4.Mortensen DS, Rodriguez AL, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Bio-org Med Chem Lett. 2001;11:2521. doi: 10.1016/s0960-894x(01)00488-7. [DOI] [PubMed] [Google Scholar]
- 5.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson KE, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. J Med Chem. 2000;43:4934. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 6.Nishiguchi GA, Rodriguez AL, Katzenellenbogen JA. Bioorg Med Chem Lett. 2002;12:947. doi: 10.1016/s0960-894x(02)00057-4. [DOI] [PubMed] [Google Scholar]
- 7.Huang YR, Katzenellenbogen JA. Org Lett. 2000;2:2833. doi: 10.1021/ol0062650. [DOI] [PubMed] [Google Scholar]
- 8.Stauffer SR, Katzenellenbogen J Comb Chem. 2000;2:318. doi: 10.1021/cc0000040. [DOI] [PubMed] [Google Scholar]
- 9.Katzenellenbogen JA, Katzenellenbogen BS, Fink BE, Stauffer SR, Mortensen DS, Sattigeri VJ, Huang Y. WO 2000019994 A1. 2000
- 10.Fink BE, Mortensen DE, Stauffer SR, Aron ZD, Katzenellenbogen JA. Chem Biol. 1999;6:205. doi: 10.1016/S1074-5521(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 11.Stauffer SR, Huang Y, Coletta CJ, Tedesco R, Katzenellenbogen JA. Bio-org Med Chem. 2001;9:141. doi: 10.1016/s0968-0896(00)00228-5. [DOI] [PubMed] [Google Scholar]
- 12.Gust R, Keilitz R, Schmidt K, von Rauch M. Arch Pharm. 2002;335:463. doi: 10.1002/ardp.200290000. [DOI] [PubMed] [Google Scholar]
- 13.Gust R, Busch S, Keilitz R, Schmidt K, von Rauch M. Arch Pharm. 2003;336:456. doi: 10.1002/ardp.200300729. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer A, Wellner A, Strauss M, Wolber G, Gust R. ChemMedChem. 2011;6:2055. doi: 10.1002/cmdc.201100283. [DOI] [PubMed] [Google Scholar]
- 15.Schaefer A, Wellner A, Gust R. ChemMedChem. 2011;6:794. doi: 10.1002/cmdc.201000537. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann J, Liebl R, von Angerer E. Steroid Biochem Molec Biol. 2005;94:57. doi: 10.1016/j.jsbmb.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann J, Von Angerer E. J Steroid Biochem Mol Biol. 2007;96:259. doi: 10.1016/j.jsbmb.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Huebner VD, Lin X, James I, Chen L, Desai M, Moore JC, Krywult B, Navaratnam T, Singh R, Trainor R, Wang L. WO 0008001 A1, 200002017
- 19.Schnurch M, Flasik R, Khan AF, Spina M, Mihovilovic MD, Stanetty P. Eur J Org Chem. 2006:3283. [Google Scholar]
- 20.Chinchilla R, Najera C. Chem Soc Rev. 2011;40:5084. doi: 10.1039/c1cs15071e. [DOI] [PubMed] [Google Scholar]
- 21.Churches QI, Stewart HE, Cohen SB, Shroder A, Turner P, Hutton CA. Pure Appl Chem. 2008;80:687. [Google Scholar]
- 22.Minkkila A, Saario SM, Kasnanen H, Leppanen J, Poso A, Nevalainen T. J Med Chem. 2008;51:7057. doi: 10.1021/jm801051t. [DOI] [PubMed] [Google Scholar]
- 23.Peng C, Zhang W, Yan G, Wang J. Org Lett. 2009;11:1667. doi: 10.1021/ol900362d. [DOI] [PubMed] [Google Scholar]
- 24.Angibaud P, Van Emelen K, Decrane L, van Brandt S, ten Holte P, Pilatte I, Roux B, Poncelet V, Speybrouck D, Queguiner L, Gaurrand S, Marien A, Floren W, Janssen L, Verdonck M, van Dun J, van Gompel J, Gilissen R, Mackie C, Du Jardin M, Peeters J, Noppe M, Van Hijfte L, Freyne E, Page M, Janicot M, Arts J. Bioorg Med Chem Lett. 2010;20:294. doi: 10.1016/j.bmcl.2009.10.118. [DOI] [PubMed] [Google Scholar]
- 25.Labaree DC, Shang J, Harris HA, O’Connor C, Reynolds TY, Hochberg RB. J Med Chem. 2003;46:1886. doi: 10.1021/jm0204340. [DOI] [PubMed] [Google Scholar]
- 26.Green S, Walter P, Kumar V, Krust A, Bonert JM, Argos P, Chambon P. Nature. 1986;320:134. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 27.Insight II, 97.2/ Discover 2.9.7. MSI Inc; San Diego: 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.