Abstract
The immune system has been reported to suppress the development and progression of neoplastic lesions; however, the exact mechanisms by which neoplastic lesions and the immune system interact are not well understood. Within the last decade, tiny membrane bound particles, approximately 30–100 nm in diameter, have been observed in the blood and other body fluids. These particles, currently called exosomes, are released from many types of tissues including tumors, and they contain and carry many proteins, and mRNAs and microRNA species. We review here how tumors suppress the immune system, especially by the formation of exosomes. Exosomes released from tumors are carried in part by the vascular system to distant cells, which phagocytose them. Depending on the proteins, mRNAs or microRNAs in the exosomes and the cell type, phagocytosis of exosomes may provide a modulating signal to the cell. In the case of exosomes from tumors, uptake of the exosomes by cells of the immune system has been reported to have three main effects: 1) suppression of the number and activity of natural killer cells, 2) suppression of the activity of T cells and 3) suppression of the number and maturation of mature dendritic cells.
Keywords: cancer, dendritic cells, exosomes, immune surveillance, intraepithelial neoplasia, NK cells, PGE2, T cells, TGFβ, TNFα
It has been suggested that the immune system modulates tumor development and progression (Zhang and Grizzle 2003); however, the extent of this modulation varies with the individual, the environment of the tumor, the type of tumor and the molecular features of the tumor. In murine models of tumors, the genetics of the mice, i.e., how close the genetics of the mice are to the genetics of the implanted tumor, affects the immune responses of mice to specific tumors (Grizzle et al. 2002, Zhang and Grizzle 2003). Because tumors develop and progress in mice and men, the immune system clearly does not inhibit completely some tumors from development and progression.
One of the strongest indications that the immune system modulates tumor development is the reported increase in development of tumors following initiation of immunity suppression for organ transplant. Following immunity suppression upon transplantation of a kidney, significant increases in number of cases of cancer have been reported in many organs and tissues. (Vajdic et al. 2006). By contrast, spontaneous resolution of tumors has proved more difficult to study. Although a few invasive melanomas have been reported to resolve either spontaneously or under conditions of increased immunity (Zhang and Grizzle 2003), such spontaneous resolution has not been studied adequately. Spontaneous regression may be much more frequent in high grade intraepithelial neoplastic lesions. Specifically, one third or more of cervical CIN3 lesions have been reported to resolve completely and an even larger proportion of CIN2 lesions regress to normal appearing epithelium (Koss 1979); however, the overall effects of the immune system on the growth, progression and resolution of neoplastic lesions is poorly understood.
Tumor surveillance
The system of immune surveillance can be subdivided into several major components that may affect or modulate neoplastic processes. These include a specific component of cellular immunity including many types of T cells and natural killer (NK) cells; a nonspecific component of cellular immunity including macrophages, dendritic cells (DC), mast cells and other granulocytes; a nonspecific component of a humoral immunity including complement, defensins, reactive molecules to cellular damage and death, and cytokines; a specific component of the humoral component with the formation of antibodies by B cells; and a specific/nonspecific communication system via circulating vesicles that carry signal molecules to specific cells and perform nonspecific functions. For example, tumors may partially escape immune surveillance by prevention of the functions of DC or subtypes of DC. This may be by prevention of maturation of DC or subtypes of DC, by effects on the infiltration of tumors by macrophages or DC, or by growth factor/cytokine inhibition of DC or their maturation, and by inhibiting the functions of DC (Vicari et al. 2002).
Factors that reduce the effectiveness of DC for immunosurveillance of tumors include VEGF, IL-6, IL-10, MCSF and PGE2. Also, whether or not tumors display HLA Class I antigens on their surface may be important to tumor progression, especially metastases (Herberman 1995, Vicari et al. 2002, Weber et al. 2007). Similarly, immunosurveillance of neoplasia is maintained by NK and T cells. A specific subtype of NK cells that expresses the neural adhesion molecule (N-CAM) and responds locally to IL-2 has been proposed to be important specifically for immunosurveillance of neoplasia (White-side and Herberman 1995).
Several reports indicate that animals and humans with specific tumors undergo suppression of specific components of their immune system; specifically, studies have reported an increase in Gr-1+ CD11b+ myeloid-derived suppressor cells in the spleen and bone marrow of animals with tumors (Gabrilovich and Nagaraj 2009). The accumulation of myeloid-derived suppressor cells (MDSCs) is associated with increased tumor burden and correlates with poor rates of survival of patients with this finding. This probably is observed because an increase in MDSCs is associated with suppression of the activation of CD4+ and CD8+ T lymphocytes as well as suppression of activation of the NK cells; specifically, the accumulation of MDSCs results in direct MDSCs -NK contact which inhibits IL-2 mediated activation of NK cells and the production of perforin by modulation of Stat5 (Liu et al. 2007).
Some proteins and other molecules that are present in cells of neoplastic lesions, especially mutated proteins, splice variants of proteins, oncofetal proteins and/or proteins modified owing to changes in post-translational modifications, e.g., reduced glycosylation, may produce an immune response when the cells of the tumor die and products from the dying cells pass into the vascular system and reach the lymphoreticular/immune system. Similarly, some of these same molecules may be expressed on the surface of tumor cells and may be recognized as “non-self” by infiltrating cells of the immune system.
Thus, the immune system likely is to be activated in patients with some neoplastic lesions by the presentation of such antigens to B-lymphocytes, T-lymphocytes and NK cells. In addition, in patients with larger cancers, there may be molecular features of hypoxia and/or other types of tissue damage that may generate signals that activate the immune system. This is indicated for many tumors by an increase in circulating acute phase reactants, e.g., reactive proteins such as transferrin and C reactive protein, and by the formation and circulation of antibodies to tumor products. These are called “tumor-specific antibodies.” Tumor-specific antibodies are of interest because their detection may be used as molecular targets for early detection of neoplasia (Chatterjee et al. 2009, Lin et al. 2007). Also, the tumor-specific antigens of tumor-specific antibodies may indicate potential targets for immune therapy. Thus, with most neoplastic processes, the immune system in patients should be activated and by some measure should inhibit the development, growth, and/or progression of neoplastic lesions.
Exosomes
Exosomes are tiny membrane particles in the blood that range in size from 40–100 nm (Clayton et al. 2001). Exosomes are produced by reverse budding of the membrane of multivesicular bodies (MVB) (Clayton et al. 2001, Hsu et al. 2003, Kapsogeorgou et al. 2005, Kim et al. 2005). Subsequently, MVB fuse with plasma membranes and release exosomes from the surfaces of cells into the extracellular space where a portion of them enter the vascular system and circulate (Clayton et al. 2001, Hsu et al. 2003, Kapsogeorgou et al. 2005, Kim et al. 2005). Release of exosomes is stimulated by calcium ionophores or phorbol esters and is inhibited by inositol 3-kinase inhibitors, e.g., wortmannin (Arita et al. 2008, Clayton et al. 2001, Lu et al. 2009). Secretion and/or production of exosomes also may be modulated by the TSAP6 protein, which is regulated by p53 (Yu et al. 2006, 2009). The turnaround time between endocytosis of a protein and secretion of an exosome containing the endocytic protein may be as short as 3 h (Thery et al. 2002). Exosomes from various types of cells may carry 300–500 common proteins (Thery et al. 2002) as well as special specific proteins that may impart different biological activities on exosomes from different sources. In some cases, the large excess of specific proteins in exosomes serves as a method by which a cell can eliminate excess or unneeded proteins (Blanc et al. 2007), especially the elimination of proteins without signal sequences that permit their secretion. The transfer of proteins outside cells by exosomes is especially important for the developing red blood cells (Blanc et al. 2007). Also, exosomes from some tissues, especially hematopoietic tissues, may express on their surfaces abundant MHC Class II and MHC Class I molecules (Admyre et al. 2007, Buschow et al. 2009, Denzer et al. 2000, Gauvreau et al. 2009, Kleijmeer et al. 2001, Lynch et al. 2009, Utsugi-Kobukai et al. 2003, Viaud et al. 2009, Vincent-Schneider et al. 2002). Because there is an excellent review that summarize biology of exosomes (Thery et al. 2009), we focus here primarily on the roles of tumor exosomes.
The circulation and absorption of exosomes into distant cells permit exosomes to influence these distant cells. The direct exposure of cells to exosomes from other cells can result in the transfer of proteins, mRNAs, and microRNAs, lipids and other molecules from one type of cell to another. This system based on exosomes is analogous to a particle based endocrine system; however, in some cases there may be actual local autocrine and paracrine signaling by exosomes.
Different cells phagocytose exosomes based on specific molecules that may coat the outer surface of the exosomal membrane. Blanc et al. (2007) designated such surface markers as “eat me signals,” and suggested that these signals are analogous to those of apoptotic cells, e.g., lysophosphatidylcholine (Blanc et al. 2007). It seems probable that one set of “eat me signals” may direct exosomes to specific target cells to provide a signal, while other sets may cause the exosomes to be cleared from the circulation by phagocytosis.
Exosomes suppress immune surveillance of tumors
It has been reported that exosomes are released from the cells of neoplastic lesions and other tissues and circulating exosomes have been suggested to be a major factor in repressing immune surveillance of tumors (Taylor et al. 2006, 2009, Taylor and Gercel-Taylor 2005). Exosomes seem to be produced by most types of cancers including melanomas, colorectal cancer, breast cancer, and prostate cancer (Andre et al. 2002b, Iero et al. 2008, Taylor and Gercel-Taylor 2005, Whiteside 2005, Wolfers et al. 2001). Exosomes from each type of cancer seem to incorporate proteins specific for their source cancers (Iero et al. 2008); specifically, melanomas produce exosomes that contain Melan A/Mart1. Exosomes not only are transferred by the vascular system, but also are present in body fluids, e.g., where tumor cells accumulate such as ascites and pleural effusions; however, the exosomes in pleural effusions tend to be larger (80 nm, mean) and have different activities (Andre et al. 2002b). Different subtypes of exosomes may perform specialized functions and may have different physical and biochemical characteristics (Poliakov et al. 2009).
Initial studies using TS/A and 4T1 murine transplantable mammary tumors in syngeneic BALB/c or nude mice demonstrated that exosomes from these tumors accelerated the growth of the same implanted tumors. One of the reported mechanisms for this increased growth is the inhibition of the number and the cytotoxic activity of NK cells; however, the viability of NK cells was not reported to be decreased by treatment with exosomes (Liu et al. 2006).
The mechanism involved in suppression of NK activity by exosomes included inhibition of the release of perforin, reduced expression of cyclin D3, and inactivation of Jak-3 mediated pathways. Exosomes from human cell lines also inhibited IL-2 stimulation of the proliferation of NK and T cells (Liu et al. 2006). The effects of exosomes from cells of the human breast cancer cell line MBA-MD-231 and a melanoma cell line on NK cells were similar (Liu et al. 2006). The ability of exosomes to inhibit T cells also depends on the Jak3 pathway and CD3-zeta.
Exosomes expressing FasL and TRAIL on their surfaces have been identified as having been produced from melanomas and colorectal carcinomas (Iero et al. 2008). Exosomes that carry active forms of FasL and TRAIL can target and induce apoptosis in activated tumor-specific T cells (Abusamra et al. 2005); however, neither FasL nor TRAIL were expressed on exosomes from TS/A cells.
The uptake of exosomes by cells and their ability to alter the function of the recipient cells has been established in several systems. For example, it has been suggested that intestinal epithelial cells and melanoma cells can secrete exosomes that can induce antigen-specific tolerance and FasL-mediated T-cell apoptosis (Abusamra et al. 2005, Kim et al. 2007, Sabapatha et al. 2006, Taylor and Gercel-Taylor 2005). Tumor exosomes also have been shown to play a role in the promotion of tumor growth by suppression of NK cell activation (Liu et al. 2006, Yu et al. 2007).
Another effect of exosomes on growth of syngenic breast tumors in mice and their progression is by a reduction of mature DC. Mature DC usually act as antigen presenting cells that on exposure to antigens from tumors become involved in the host antitumor immune response. When immature DC are treated with exosomes from TS/A murine breast tumors, maturation of the Gr-1+, CD11b+ cells was blocked. The time course of this response is important, because blockage of the maturation of DC by TS/A exosomes was effective at 0 and 3 days after GM-CSF administration, but not after 5 days (Liu et al. 2006, Yu et al. 2007). IL-6 and phosphorylated Stat3 also were increased in an exosome dose-dependent manner when Gr-1+ CD11b+ cells were stimulated with exosomes. Il-6 and its stimulation of pStat3 usually produce an oncogenic cytokine response. This effect in IL-6 may also be time-dependent and IL-6 also may be involved in blocking the maturation of CD11b+ cells (Liu et al. 2006, Yu et al. 2007). Tumor exosomes also promote the induction of myeloid suppressor cells to inhibit tumor cytotoxicity of T cells. Furthermore, MDSC mediated promotion of tumor progression in vivo is dependent on Tumor exosome prostaglandin E2 (PGE2) and TGF-beta molecules (Xiang et al. 2009). Conversely, the exosomes secreted by DC can stimulate responses of T cells against tumor growth and increase the longevity of Ag presentation by DC (Andre et al. 2002a, Luketic et al. 2007, Zitvogel et al. 1998). DC derived exosomes also may activate NK cells, which leads to tumor regression (Viaud et al. 2009). These antitumor effects were antigen-specific and were associated with the activity of T cells. It is conceivable that the distinct functions of tumor-derived vs. DC-derived exosomes depend on the composition of the exosomes. It is likely that taking up exosomes may affect the multiple pathways of the recipient cells (Mathivanan and Simpson 2009).
In addition to reducing immune surveillance, exosomes also may reduce the effectiveness of some, but not all, therapeutic drugs. For example, exosomes were reported to eliminate doxorubicin from cancer cells by packaging it and other therapeutic drugs in exosomes, then eliminating them within exosomes (Shedden et al. 2003).
Exosomes and the immune system in autoimmune diseases
The importance of exosomes in regulation of the immune system is demonstrated by their effects in specific autoimmune diseases. For example, treatment of DC with IL-10 has been reported to generate exosomes that can prevent inflammation and arthritis induced by collagen II injections in rabbits and mice (Kim et al. 2005). Exosomes of the epithelial cells of salivary glands have been reported to contain a great variety of autoantigens and these exosomes are postulated to function in antigen presentation to either induce T cell tolerance or generate an autoimmune response (Hsu et al. 2003, Kapsogeorgou et al. 2005). Also, exosomes have been reported to be a source of antigens for antigen presenting cells as these cells present antigens to T lymphocytes and NK cells (Clayton et al. 2001, Vincent-Schneider et al. 2002).
Fibroblasts obtained from patients with rheumatoid arthritis (RA), but not from other forms of arthritis, have been reported to generate exosomes that contain TNFα and these exosomes can kill TNFα sensitive cells (Zhang et al. 2006); the killing of cells sensitive to TNFα has been reported to be blocked by treatment with soluble TNFαR1. Exosomes from RA patients also have been reported to induce the phosphorylation of Akt, to increase NF-κB expression in T cells and to reduce apoptosis of activated T cells. Because these pathways are involved in the pathogenesis of RA, exosomes from fibroblasts from RA patients could play a role in improvement or relapse of RA. By contrast, exosome-like particles from thymic cells are reported to induce CD4+, CD25− T cells to form CD4+, CD25+, Foxp3+ T regulatory cells (Treg) in peripheral tissues (Wang et al. 2008) that participate in the maintenance of immune tolerance. The specific exosomal protein in exosome-like particles important to this transition is TGFβ, which functions to produce T regulatory cells.
Potential therapeutic approaches to suppression of the immune system by exosomes
Because exosomes are so involved in the modulation of the immune system in patients with neoplastic lesions and because the production of exosomes may reduce the effectiveness of molecular therapy for cancer, exosomes offer a potential pathway or target to improve therapy for neoplasia. It has been suggested that drugs that affect the pathways of exocytosis may be used to decrease the release of exosomes and thus potentially release the inhibition of the immunosurveillance system or to improve therapy with specific drugs (Iero et al. 2008).
Curcumin is a polyphenol that can be isolated from the spice, tumeric. It has been reported to have anticancer activity and to block proteosome activity; thus, it was tested for its ability to block the effects of exosomes on the immune system. Curcumin in concentrations between 0.2 and 1.0 μM inhibited the suppression of NK cell cytotoxicity by exosomes. The response to curcumin was dose-dependent. Similarly, curcumin in concentrations between 0.2 and 1 μM inhibited in a dose-dependent manner the exosome-induced blockage of proliferation of NK cells in response to IL-2. Curcumin treatment also resulted in dissemination of exosomal proteins and lowered the inhibition of Jak3 and p-Stat5 caused by exosomes (Zhang et al. 2007).
The use of exosomes to improve the delivery of tumor antigens for vaccines has been suggested by Hsu et al. (2003). Because DC have MHC-I and MHC-II molecules as well as CD86, a co-stimulatory molecule for T cells, purified exosomes can be loaded with multiple antigenic peptides in very high amounts. This approach can stimulate CD8+ cytotoxic T cells as well as CD4+ helper cells to produce an effective anti-tumor T cell response (Hsu et al. 2003).
More recently, encouraging studies have indicated that the DC-derived exosomes activate NK cells in melanoma patients after vaccination with DC-derived exosomes in phase I trials (Viaud et al. 2009). Similarly, exosomes have been used in clinical trials for patients with colorectal cancer and metastatic melanoma. Also, ascites-derived exosomes combined with GM-CSF have been used for immunotherapy of advanced colorectal cancer (Dai et al. 2008). A number of strategies have been reported to improve the antigenicity of tumor exosomes. For example, using heat-shocked exosomes has been shown to increase anti-tumor immune responses in therapeutic models of lymphoma in vivo (Chen et al. 2006). T-cell-derived exosomes, surface anchored with superantigen staphylococcal enterotoxin A tailed with a highly hydrophobic transmembrane domain, can efficiently induce tumor-specific T cell responses in mice (Xiu et al. 2007). Also, CpG oligomeric sequences or double stranded RNA used as adjuvants bind on DC to T-cell-like receptor g, which causes activation and maturation of DC. Also, use of CpG adjuvants with exosomes may induce a cytotoxic T lymphocyte response (Chaput et al. 2004).
Unanswered issues and future directions
Much has been learned concerning how exosomes mediate cell to cell communication. Recent findings indicate that the exosomes function beyond suppressing immune responses. Indeed, comparisons of whole tumor cell lysates with tumor exosomes often reveal striking enrichment within the exosomes of cell-specific tumor antigens including HER2/neu, melan-A, Silv, carcinoembryonic antigen, mesothelin, and many other antigens. Immunization of naïve mice with DC pulsed with cancer cell–derived exosomes demonstrates that it is possible to induce protective antitumor immune responses. This aspect is being explored in clinical studies (Dai et al. 2008, Escudier et al. 2005, Wolfers et al. 2001).
Although DC pulsed with tumor exosomes can stimulate cytotoxic T cells to kill tumor cells, cytotoxic T cell-mediated killing of tumor cells may not take place in vivo, i.e., in murine tumor models or cancer patients. It is important to emphasize that the cancer exosomes may not be taken up by mature DC. By contrast, exosomes are taken up by immature myeloid precursors, which blocks their differentiation and maturation. Therefore, a challenging question is how to redirect tumor exosomes from immature myeloid precursor cells to mature DC. Also, because tumor exosomes contain both potential tumor antigens and molecules involved in immunosuppression, for exosomes to be used in approaches to tumor vaccination, immune suppression molecules must either be eliminated from exosomes or their effects minimized. We also hypothesize that factors derived from the microenvironment of tumors are important to the composition of exosomes and thus to their biological effects on recipient cells. Addressing this important issue poses several challenges. One of these requires the development of techniques to purify the exosomes from tumor tissues at different stages. Another major challenge to understanding the pathological functions of exosomes from tumors is the need to identify precisely how various molecules, e.g., proteins, mRNAs, microRNAs, are sorted within exosomes, the pathways involved in such sorting, and the microenvironmental factors affecting the sorting. Also, we must determine whether and how selective molecules of the contents of exosomes are delivered to specific types of cells and how the cells process such signals selectively.
Exosomes no longer are considered only a molecular waste disposal pathway, but also an endocrine pathway that is crucial for cell to cell communication. When our understanding of exosomes is complete, instead of relegating exosomes to inhibiting the maturation of DC precursors, blocking the activities of NK cells, and blocking the cytotoxicity of T cells, the target cells of exosomes and their functions in cellular biology or communication may be re-directed to stimulating host responses against tumors to inhibit the development, growth and progression of specific tumors.
Exosomes may be produced by all types of tumor cells and may carry many types of signal molecules including mRNA, microRNAs, proteins and peptides, and they can remove therapeutic drugs and unneeded endogenous molecules. Exosomes may cause common events including suppression of NK cell cytotoxicity and IL-2 induced NK cellular proliferation, and inhibition of the maturation of DC and monocytes (CD14+). In this way, exosomes may greatly reduce the number of effective antigen presenting cells and inhibiting T cell responses. All these effects reduce the effectiveness of the immune surveillance system for targeting neoplastic lesions (Fig. 1). Future therapy for tumors may focus on decreasing the release of exosomes or the use of exosomes for vaccination against neoplastic lesions.
Fig. 1.
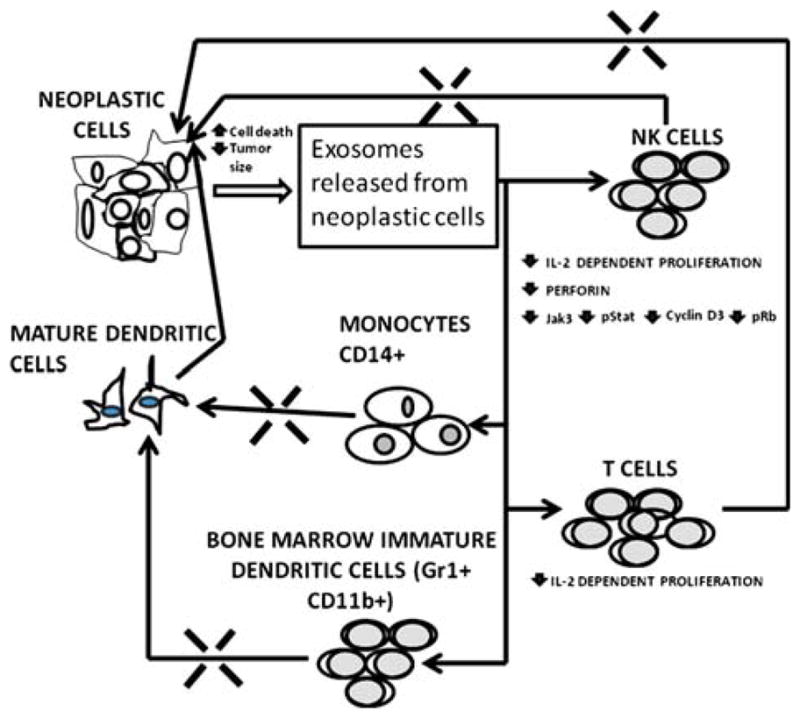
Demonstration of how neoplastic lesions inhibit the immune system by the release of exosomes. Exosomes inhibit the number and actions of NK cells shown by the blockage of the pathway between NK cells and the tumor. Similarly, exosomes inhibit the number of T cells that can infiltrate tumors. Also shown are the interaction of exosomes with monocytes and immature DC, which prevents both from forming mature DC. The decrease in DC, NK cells and T cells in the tumor causes a reversal in the cell death usually caused by these cells and results in a decrease in immune system-induced death of neoplastic cells and growth of the tumor.
Acknowledgments
Supported in part by the grant for the Early Detection Reference Laboratory at UAB of the Early Detection Research Network 5U24CA086359-10 to WEG, National Institutes of Health grant numbers RO1CA116092, RO1CA107181, RO1AT004294, R01CA137037 to HGZ, Birmingham Veterans Administration Medical Center Merit Review Grants to HGZ, and the Susan G. Komen Breast Cancer Foundation grants BCTR0707323 to HGZ and BCTR0600484 to WEG.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35:169–173. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, Gabrielsson S. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allerg Clin Immunol. 2007;120:1418–1424. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- André F, Schartz NE, Chaput N, Flament C, Raposo G, Amigorena S, Angevin E, Zitvogel L. Tumor-derived exosomes: a new source of tumor rejection antigens. Vaccine. 2002a;20(Suppl 4):A28–A31. doi: 10.1016/s0264-410x(02)00384-5. [DOI] [PubMed] [Google Scholar]
- André F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002b;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- Arita S, Baba E, Shibata Y, Niiro H, Shimoda S, Isobe T, Kusaba H, Nakano S, Harada M. B cell activation regulates exosomal HLA production. Eur J Immunol. 2008;38:1423–1434. doi: 10.1002/eji.200737694. [DOI] [PubMed] [Google Scholar]
- Blanc L, Barres C, Bette-Bobillo P, Vidal M. Reticulocyte-secreted exosomes bind natural IgM antibodies: involvement of a ROS-activatable endosomal phospholipase iPLA2. Blood. 2007;110:3407–3416. doi: 10.1182/blood-2007-04-085845. [DOI] [PubMed] [Google Scholar]
- Buschow SI, Nolte-’t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, Ossendorp F, Melief CJ, Raposo G, Wubbolts R, Wauben MH, Stoorvogel W. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10:1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- Chaput N, Taïeb J, Schartz NEC, André F, Angevin E, Zitvogel L. Exosome-based immunotherapy. Cancer Immunol Immunother. 2004;53:234–239. doi: 10.1007/s00262-003-0472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Wojciechowski J, Tainsky MA. Discovery of antibody biomarkers using protein micro-arrays of tumor antigens cloned in high throughput. Methods Mol Biol. 2009;520:21–38. doi: 10.1007/978-1-60327-811-9_3. [DOI] [PubMed] [Google Scholar]
- Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, Cao X. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol. 2006;36:1598–1607. doi: 10.1002/eji.200535501. [DOI] [PubMed] [Google Scholar]
- Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA, Newman GR, Jasani B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods. 2001;247:163–174. doi: 10.1016/s0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165:1259–1265. doi: 10.4049/jimmunol.165.3.1259. [DOI] [PubMed] [Google Scholar]
- Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvreau ME, Côté MH, Bourgeois-Daigneault MC, Rivard LD, Xiu F, Brunet A, Shaw A, Steimle V, Thibodeau J. Sorting of MHC class II molecules into exosomes through a ubiquitin-independent pathway. Traffic. 2009;10:1518–1527. doi: 10.1111/j.1600-0854.2009.00948.x. [DOI] [PubMed] [Google Scholar]
- Grizzle WE, Mountz JD, Yang PA, Xu X, Sun S, Van Zant GE, Williams RW, Hsu HC, Zhang HG. BXD recombinant inbred mice represent a novel T cell-mediated immune response tumor model. Int J Cancer. 2002;101:270–279. doi: 10.1002/ijc.10606. [DOI] [PubMed] [Google Scholar]
- Herberman RB. Potential role of natural antibodies in resistance to tumor growth. Nat Immun. 1995;14:1. [PubMed] [Google Scholar]
- Hsu DH, Paz P, Villaflor G, Rivas A, Mehta-Damani A, Angevin E, Zitvogel L, Le Pecq JB. Exosomes as a tumor vaccine: enhancing potency through direct loading of antigenic peptides. J Immunother. 2003;26:440–450. doi: 10.1097/00002371-200309000-00007. [DOI] [PubMed] [Google Scholar]
- Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. Salivary gland epithelial cell exosomes: A source of autoantigenic ribonucleoproteins. Arthritis Rheum. 2005;52:1517–1521. doi: 10.1002/art.21005. [DOI] [PubMed] [Google Scholar]
- Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. MHC class II+ exosomes in plasma suppress inflammation in an antigen-specific and Fas ligand/Fas-dependent manner. J Immunol. 2007;179:2235–2241. doi: 10.4049/jimmunol.179.4.2235. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- Kleijmeer MJ, Escola JM, UytdeHaag FG, Jakobson E, Griffith JM, Osterhaus AD, Stoorvogel W, Melief CJ, Rabouille C, Geuze HJ. Antigen loading of MHC class I molecules in the endocytic tract. Traffic. 2001;2:124–137. doi: 10.1034/j.1600-0854.2001.020207.x. [DOI] [PubMed] [Google Scholar]
- Koss LG. Diagnosis of early endometrial cancer and precancerous states. Ann Clin Lab Sci. 1979;9:189–194. [PubMed] [Google Scholar]
- Lin F, Zhang PL, Yang XJ, Shi J, Blasick T, Han WK, Wang HL, Shen SS, Teh BT, Bonventre JV. Human kidney injury molecule-1 (hKIM-1): a useful immunohistochemical marker for diagnosing renal cell carcinoma and ovarian clear cell carcinoma. Am J Surg Pathol. 2007;31:371–381. doi: 10.1097/01.pas.0000213353.95508.67. [DOI] [PubMed] [Google Scholar]
- Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, Zhang HG. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE, Zhang HG. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- Lu Q, Zhang J, Allison R, Gay H, Yang WX, Bhowmick NA, Frelix G, Shappell S, Chen YH. Identification of extracellular delta-catenin accumulation for prostate cancer detection. Prostate. 2009;69:411–418. doi: 10.1002/pros.20902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luketic L, Delanghe J, Sobol PT, Yang P, Frotten E, Mossman KL, Gauldie J, Bramson J, Wan Y. Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. J Immunol. 2007;179:5024–5032. doi: 10.4049/jimmunol.179.8.5024. [DOI] [PubMed] [Google Scholar]
- Lynch S, Santos SG, Campbell EC, Nimmo AM, Botting C, Prescott A, Antoniou AN, Powis SJ. Novel MHC class I structures on exosomes. J Immunol. 2009;183:1884–1891. doi: 10.4049/jimmunol.0900798. [DOI] [PubMed] [Google Scholar]
- Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate. 2009;69:159–167. doi: 10.1002/pros.20860. [DOI] [PubMed] [Google Scholar]
- Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56:345–355. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer. 2005;92:305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput N, Taïeb J, Schartz NEC, André F, Angevin E, Zitvogel L. Exosome-based immunotherapy. Cancer Immunol Immunother. 2004;53:234–239. doi: 10.1007/s00262-003-0472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C, Parker LP. Patient-derived tumor-reactive antibodies as diagnostic markers for ovarian cancer. Gynecol Oncol. 2009;1:112–120. doi: 10.1016/j.ygyno.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol Lett. 2003;89:125–131. doi: 10.1016/s0165-2478(03)00128-7. [DOI] [PubMed] [Google Scholar]
- Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, Chaput N. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One. 2009;4:e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari AP, Caux C, Trinchieri G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin Cancer Biol. 2002;12:33–42. doi: 10.1006/scbi.2001.0400. [DOI] [PubMed] [Google Scholar]
- Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, Bonnerot C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14:713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Liu Y, Qin A, Shah SV, Deng ZB, Xiang X, Cheng Z, Liu C, Wang J, Zhang L, Grizzle WE, Zhang HG. Thymus exosomes-like particles induce regulatory T cells. J Immunol. 2008;181:5242–5248. doi: 10.4049/jimmunol.181.8.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K, Doucet M, Kominsky S. Renal cell carcinoma bone metastasis-elucidating the molecular targets. Cancer Metastasis Rev. 2007;26:691–704. doi: 10.1007/s10555-007-9090-y. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. Tumour-derived exosomes or micro-vesicles: another mechanism of tumour escape from the host immune system? Br J Cancer. 2005;92:209–211. doi: 10.1038/sj.bjc.6602360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL, Herberman RB. The role of natural killer cells in immune surveillance of cancer. Curr Opin Immunol. 1995;7:704–710. doi: 10.1016/0952-7915(95)80080-8. [DOI] [PubMed] [Google Scholar]
- Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, Grizzle WE, Mobley J, Zhang HG. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu F, Cai Z, Yang Y, Wang X, Wang J, Cao X. Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes. J Mol Med. 2007;85:511–521. doi: 10.1007/s00109-006-0154-1. [DOI] [PubMed] [Google Scholar]
- Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, Falkson C, Zhang HG. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- Yu X, Riley T, Levine AJ. The regulation of the endosomal compartment by p53 the tumor suppressor gene. FEBS. 2009;276:2201–2212. doi: 10.1111/j.1742-4658.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- Zhang HG, Grizzle WE. Aging, immunity, and tumor susceptibility. Immunol Allergy Clin North Am. 2003;23:83–102. vi. doi: 10.1016/s0889-8561(02)00085-1. [DOI] [PubMed] [Google Scholar]
- Zhang HG, Kim H, Liu C, Yu S, Wang J, Grizzle WE, Kimberly RP, Barnes S. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta. 2007;1773:1116–1123. doi: 10.1016/j.bbamcr.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HG, Liu C, Su K, Yu S, Zhang L, Zhang S, Wang J, Cao X, Grizzle W, Kimberly RP. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol. 2006;176:7385–7393. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]


