Fig. 4.
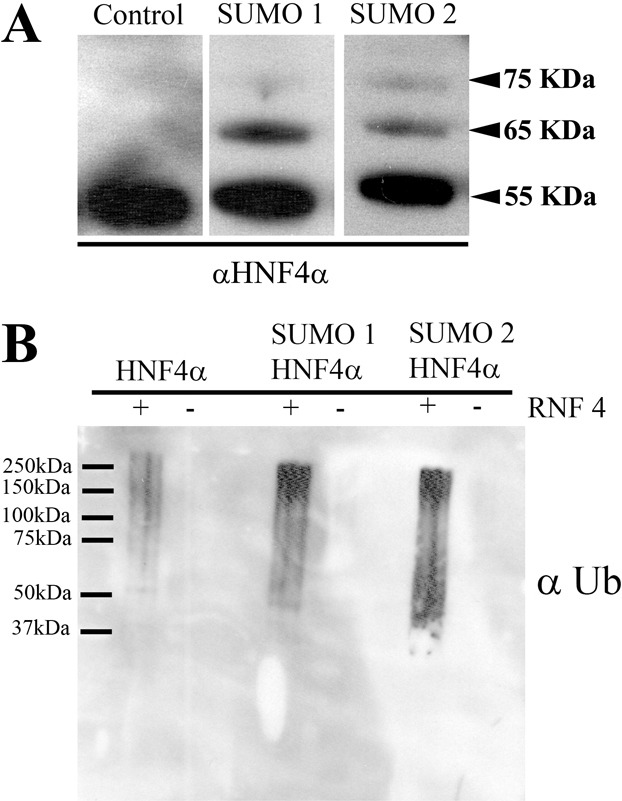
PolySUMOylation of HNF4α enhances RNF4-mediated ubiquitination in vitro. (A) In order to study HNF4α ubiquitin modification in vitro we employed an in vitro ubiquitination assay. WT HNF4α was mono- and polySUMOylated using SUMO1 and SUMO2 but absent from the control lane. (B) SUMOylated HNF4α protein was incubated with ubiquitination machinery (E1 and E2) in the presence and absence of RNF4 (E3). Following assay completion, HNF4α was immunoprecipitated and ubiquitin conjugation analysed using western blotting. Ubiquitination only occurred in the presence of RNF4 and was increased when HNF4α was polySUMOylated. (C) Densitometry analysis was performed using ImageJ software. The level of ubiquitination was increased more than sixfold over the control when HNF4α was polySUMOylated and twofold when HNF4α was monoSUMOylated.
