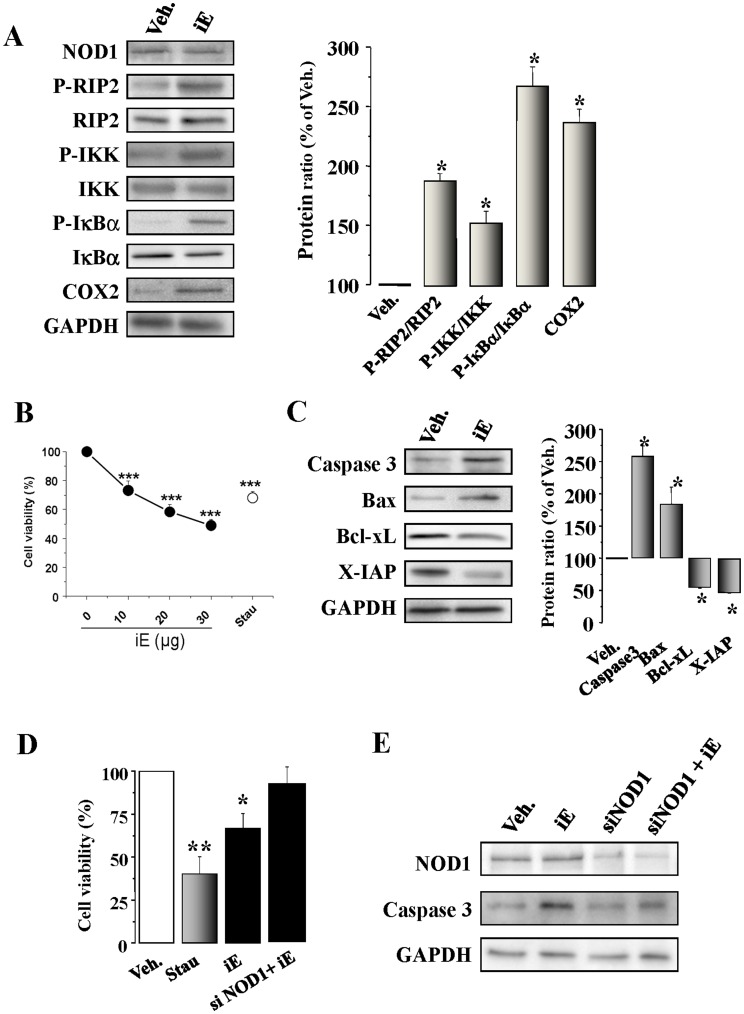
Figure 5. The NOD1 agonist iE induces NF-κB activation and apoptosis in cardiomyocytes and H9c2 cells. Effect of NOD1 ablation with siRNAs.
(A) Native murine cardiomyocytes were incubated for 15 to 60 min, or 48 h with 20 µg/ml of iE. iE treatment induces an up-regulation of P-RIP2/RIP2, P-IKK/IKK, P-IκBα/IκBα (15–60 min) and COX2 (48 h) protein levels. (B) iE induces a decrease in cardiomyocyte viability in a dose-response form. Cardiomyocytes incubated for 2 h with 10–30 µg/ml of iE; staurosporine (Stau, 100 ng/ml) were used as a positive control to induce apoptosis. (C). iE treatment induces an up-regulation of caspase 3 and Bax protein levels and down-regulates the BcL-xL and X-IAP protein levels. (D) iE or Stau administration for 24 h in H9c2 promoted a decrease in viability as determined by MTT activity. NOD1 suppression by siRNA prevented the effect of iE on cell viability. (E) Representative blot of NOD1, caspase 3 and GAPDH obtained in vehicle and iE treated cells, NOD1 siRNAs (siNOD1) and NOD1 siRNA+iE in H9c2 cells. All targets protein levels were normalized by GAPDH. Data are expressed in histograms representing the mean±SEM vs. vehicle (100%); n = 3–5 samples per condition.*p<0.05, **p<0.01 and ***p<0.001 vs. vehicle.