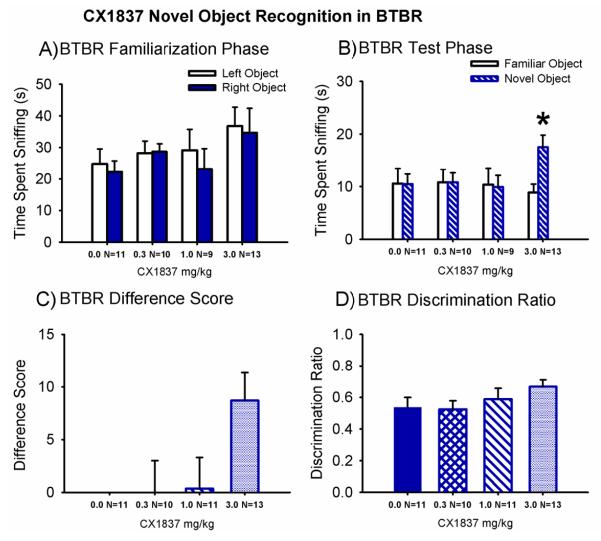
Figure 7. CX1837 increased novel object recognition in BTBR.
Novel object recognition was assayed in a Plexiglas arena, after vehicle (100 mg peanut butter pellet) or CX1837 treatment. The testing sequence consisted of a 60 minute habituation session in the empty arena, a 10 minute familiarization session in which two identical objects were placed on the floor of the arena, and a 5 minute test session in which one previous object and one new object was placed on the floor of the arena. No innate object preference or side bias was observed during the familiarization session (A), in BTBR mice treated with vehicle (100 mg peanut butter pellet) or CX1837. (B) BTBR failed to exhibit novel object recognition after treatment with vehicle and CX1837 (0.3 mg/kg and 1.0 mg/kg), consistent with previously reported cognitive deficits in BTBR mice. CX1837, 3.0 mg/kg, rescued the deficit. (C) A commonly used derived parameter, object recall difference, demonstrated scores ~ 0 for the vehicle and CX1837 (0.3 mg/kg and 1.0 mg/kg) treatment groups. A trend (p = 0.09) for a significant novel object recall measured by difference score was observed in BTBR treated at the 3.0 mg/kg dose of CX1837. (D) Another commonly used derived parameter, the object recall discrimination ratio, showed absence of novel object recognition, ratios ~ 0.5, for vehicle and CX1837 (0.3 mg/kg and 1.0 mg/kg) treatment groups. A trend (p = 0.09) for a significant novel object recall by discrimination ratio was observed for BTBR treated with the 3.0 mg/kg dose. *p < 0.05, novel object versus familiar object. N = 9-13 per dose for each strain.