Figure 11.
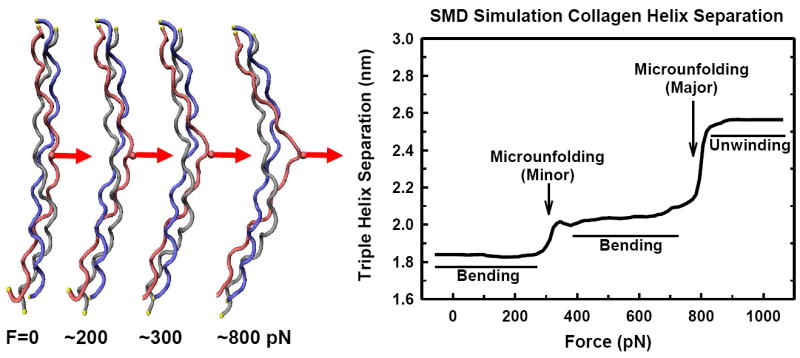
Force-induced microunfolding of the collagen triple helix [49]. Steered molecular dynamic (SMD) simulation of triple helical peptide deformation from a perpendicularly (transversely) applied mechanical force to a single α-chain (amino acid side chain, red). The triple helix first bends with local triple helix microunfolding (~300 pN). Bending continues at higher forces (~400-800 pN) without significant separation of the individual chains. Fundamental mechanics principles show that tumor growth and expansion would cause increasing tension in the surrounding collagen (see Fig. 14), suggesting that this tension likely assists in trapping and containment at early stages of cancer cell proliferation by preventing matrix degradation. At some point however, the enzymes begin to degrade the collagen and the tumor cells are now able to pass through the surrounding stroma. One potential contributing mechanism may be due to increasing cellular mechanical forces being triggered through process such as TGFβ signaling and upregulation of α-smooth muscle actin. As cellular forces on the matrix increase, SMD data shows that local helix disruption would be induced thus facilitating MMP proteolytic activity against the otherwise previously protected collagen. At forces greater then ~900 pN the α-chains separate with loss of the triple helix conformation (α-chain destabilization). In this simulation, all three chains (gray, red, blue) are fixed at both ends of the triple helix.
