Abstract
Malignant pleural mesothelioma (MPM) is a highly aggressive neoplasm primarily arising from surface serosal cells of the pleura and is strongly associated with asbestos exposure. Patients with MPM often develop pleural fluid as initial presentation. However, cytological diagnosis using pleural fluid is usually difficult and has limited utility. A useful molecular marker for differential diagnosis particularly with lung cancer (LC) is urgently needed. The aim of the present study was to investigate the diagnostic value of soluble mesothelin-related protein (SMRP) in pleural fluid. Pleural fluids were collected from 23 patients with MPM, 38 with LC, 26 with benign asbestos pleurisy (BAP), 5 with tuberculosis pleurisy (TP) and 4 with chronic heart failure (CHF), and the SMRP concentration was determined. All data were analyzed by using non-parametric two-sided statistical tests. The median concentration of SMRP in MPM, LC, BAP, TP and CHF were 11.5 (range 0.90–82.80), 5.20 (0.05–36.40), 6.65 (1.45–11.25), 3.20 (1.65–6.50) and 2.03 (1.35–2.80) nmol/l, respectively. The SMRP concentration was significantly higher in MPM than in the other diseases (P=0.001). The area under the ROC curve (AUC) values of the MPM diagnosis was 0.75 for the differential diagnosis from the other groups. Based on the cut-off value of 8 nmol/l, the sensitivity and specificity for diagnosis of MPM were 70.0 and 68.4%, respectively. These results indicate that the SMRP concentration in pleural fluid is a useful marker for the diagnosis of MPM.
Keywords: mesothelin, mesothelioma, asbestos
Introduction
Malignant pleural mesothelioma (MPM) is a highly aggressive tumor with a poor survival rate that arises from the surface cells of the pleura. It is a rare tumor; however, MPM has become a very serious public health concern in Japan. A newspaper article, published in June 2005, reported that five residents who had lived near a now-closed asbestos cement pipe plant in Amagasaki, Japan, developed pleural mesothelioma (1). The industrial use of asbestos has been banned in Japan since 2006, but the incidence of MPM is expected to continue increasing for the next few decades due to the past usage of asbestos (2).
MPM has therapeutic and diagnostic challenges. Surgical resection, often combined with radiotherapy or adjuvant chemotherapy, is indicated for the treatment of MPM in the earlier stage. There is a small population of patients who achieve prolonged disease-free survival. Yet the majority of cases are already progressive at the time of diagnosis, and these patients exhibit an extremely poor prognosis (3). Systemic chemotherapy or radiotherapy to date has not had an impact on patient survival for advanced cases. Thus, it is quite important to diagnosis MPM at an early stage. Most MPM cases demonstrate pleural effusion at the time of diagnosis, but cytological diagnosis with pleural effusion is usually difficult and has limited utility. To obtain a definite diagnosis, a thoracoscopic or percutaneous biopsy should be performed to obtain adequate specimens for pathological and immunohistochemical analyses. Yet, even with these procedures, it is sometimes difficult to differentiate MPM from other pleural diseases including benign asbestos pleurisy (BAP), tuberculosis pleurisy (TP), or pleural metastasis of lung cancer (LC). Several investigators have sought to improve the differential diagnosis of pleural effusion by measuring tumor markers. Shi et al reported the usefulness of measuring the pleural carcinoembryonic antigen for the diagnosis of malignant pleural effusion (4). Similar findings were reported regarding cytokeratin 19 fragment 21-1 and carbohydrate antigen (CA) 125, CA15-3 and CA19-9 (5). Aoe et al previously reported that the concentration of receptor-binding cancer antigen expressed on Siso cells (RCAS1) was higher in malignant pleural effusion than in non-malignant effusion (6), but the usefulness of these markers has not yet been fully established in clinical practice. A useful molecular marker for the differential diagnosis of these diseases is therefore urgently needed.
Mesothelin is a 40-kDa cell surface glycosylated phosphatidylinositol (GPI)-anchored glycoprotein which has putative functions in cell-to-cell adhesion (7). Mesothelin is expressed on normal mesothelial cells (8); however, it is highly overexpressed in cancers such as MPM (9,10), pulmonary carcinomas (11–14) and other neoplasms (15,16). Soluble mesothelin-related protein (SMRP) is recognized as a cleaved fragment of membrane-bound mesothelin (17). Robinson and colleagues reported that serum SMRP levels were elevated in MPM when compared with healthy asbestos-exposed and non-exposed subjects, and with other pulmonary diseases including LC (18). Similar results were reported by Cristaudo et al (19) and Schneider et al (20) who demonstrated that SMRP blood concentrations were significantly higher in MPM than in LC cases. These findings suggest the usefulness of serum SMRP as a diagnostic or screening marker of MPM.
The SMRP value in pleural fluid was evaluated by Scherpereel et al (21) and Pass et al (22). Both research groups reported that the pleural SMRP value was higher than that in serum, and the level was higher in MPM than in other pulmonary diseases. Therefore, the aim of the present study was to investigate the SMRP level in pleural fluid in Japanese patients with MPM. For this purpose, SMRP concentrations in pleural fluid from Japanese patients with MPM were examined and compared with those of patients with BAP, TP or LC. Correlations between SMRP and asbestos exposure were also examined.
Materials and methods
Materials
Pleural fluid was collected from patients with MPM. For these cases, pathological diagnosis of MPM was confirmed based on standard H&E staining and positive immunohistochemical reactivity to mesothelial markers such as calretinin, Wilms’ tumor 1, or thrombomodulin, and negative reactivity to carcinoembryonic antigen. The clinical stage of MPM was determined according to the International Mesothelioma Interest Group (IMIG) criteria (23) and was based on staging procedures including computed tomographic (CT) scans of the chest and abdomen, magnetic resonance images of the brain and Technetium-99m hydroxymethylene diphosphonate bone scans. Survival data of the patients with MPM were determined from the day of diagnosis to the day of death or last follow-up. Pleural fluid was also collected from patients with LC, BAP, TP and with chronic heart failure (CHF) as controls. LC was diagnosed in cases where lung cancer cells were detected in the pleural effuion. Histological subtypes of LC were based on the World Health Organization (WHO) classification (24). The clinical stage of the disease was assessed using the International Staging System (25). TP was diagnosed in cases in which Mycobacterium tuberculosis was detected in the pleural fluid. TP was also diagnosed in cases with higher concentrations of adenosine deaminase (>50 IU/l) and when lymphocyte dominancy was shown in the fluid. CHF was diagnosed in cases which demonstrated transudate fluid with known cardiac diseases. The diagnosis of BAP was determined by exclusion of other specific causes in patients with past asbestos exposure, in which malignant diseases were ruled out with thoracoscopy. Informed consent was provided by all patients, and the study was conducted with approval of the appropriate institutional review boards.
SMRP measurement
SMRP was measured using the chemiluminescent enzyme immunoassay (CLEIA) (Fujirebio Diagnostics, Malvern, PA, USA) based on the 2-step sandwich method. In brief, 20 μl of sample was mixed with 180 μl of sample diluents, then 20 μl of the diluted sample was incubated with 250 μl of anti-SMRP antibody-coated ferrite particles at 37°C for 10 min. After washing, 250 μl of anti-SMRP antibodies coupled with alkaline phosphate was added and incubated at 37°C for 10 min. After a washing step, 200 μl of substrate [3-(2′-spiroadamantane)-4-methoxy-4-(3″-phosphoryloxy) phenyl-1,2-dioxetane disodium salt; AMPPD] solution was added, followed by incubation at 37°C for 5 min. Luminescence at a wavelength of 477 nm was measured, and the SMRP concentration of each sample was calculated with the standard curve method.
Asbestos body burden
Quantification of asbestos bodies was performed using the protocol modified by Kohyama and Suzuki (26). In brief, portions of paraffin-embedded normal lung tissue (1–2 g) obtained from surgery or autopsy were deparaffinized with xylene, then microcut. These were digested with solution containing 5–20% sodium hypochlorite and KOH for 6 h at 60°C. Following digestion, samples were pelleted and resuspended in distilled water. Samples were then mixed well and filtered through a cellulose ester membranous filter which was dehydrated and cut in half. Pieces of the filter were mounted on microscope slides and dried with acetone vapor. Asbestos bodies were then counted, and the asbestos bodies per (wet weight) gram of lung were calculated.
Statistical analyses
Comparisons between groups were performed using the Kruskal-Wallis test and non-parametric analysis using the Mann-Whitney U test. Areas under receiver operating curves (ROC) were calculated using standard techniques. Survival data were determined from the day of diagnosis to the day of death or last follow-up and analyzed based on the Kaplan-Meyer method. Correlations between pleural SMRP values and asbestos body or patient survival were calculated based on Pearson’s correlation coefficient (PCI). Statistical calculations were performed with SPSS Statistical Package version 11.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Between January 2004 and July 2007, pleural fluids were collected from 23 patients with MPM, 38 with LC, 26 with BAP, 5 with TP and 4 with CHF at the Okayama Rosai Hospital. Of the 23 cases (median age 64 years; range 47–89; male/female 21/2) diagnosed with MPM, there were 15 epithelioid, 2 biphasic, 4 sarcomatoid and 2 unknown pathological subtypes. According to the IMIG staging system, there were 3 cases in stage I, 2 in stage II, 9 in stage III, 6 in stage IV and 3 unknown. Of the 38 cases (median age 69.5 years; range 46–91; male/female 29/9) diagnosed with LC, there were 24 patients with adenocarcinoma, 4 with small-cell carcinoma, 3 with squamous cell carcinoma and 7 undetermined pathological subtypes. The characteristics of the patients are summarized in Table I.
Table I.
Patient characteristics.
| MPM | PMLC | BAP | TP | CHF | |
|---|---|---|---|---|---|
| No. | 23 | 38 | 26 | 5 | 4 |
| Age (years) | |||||
| Median (range) | 64 (47–89) | 70 (48–90) | 75.5 (58–88) | 82 (68–88) | 74 (68–82) |
| Gender | |||||
| Male/Female | 21/2 | 28/10 | 26/0 | 5/0 | 3/1 |
| Asbestos exposure period (years) | |||||
| Median (range) | 33 (5–51) | - | 30 (3–46) | - | |
| Histology | |||||
| Epithelioid | 15 | - | - | - | |
| Biphasic | 2 | - | - | - | |
| Sarcomatoid | 4 | - | - | - | |
| Unknown | 2 | - | - | - | |
| Adenocarcinoma | 24 | ||||
| Squamous cell carcinoma | 3 | ||||
| Small-cell carcinoma | 4 | ||||
| Not determined | 7 | ||||
| Stage | |||||
| I | 3 | - | - | - | |
| II | 2 | - | - | - | |
| III | 9 | - | - | - | |
| IV | 6 | - | - | - | |
| Unknown | 3 | - | - | - |
MPM, malignant pleural mesothelioma; PMLC, pleural metastasis of lung cancer, BAP, benign asbestos pleurisy; TP, tuberculosis pleurisy; CHF, chronic heart failure.
SMRP value in MPM
According to the clinical stage and pathological subtypes of MPM, a trend was noted in which the SMRP value was higher in advanced stages (III and IV, n=16; median 13.8, range 2.85–82.8 nmol/l) compared with the value in early stages (I and II, n=5; median 7.9, range 2.5–33.9 nmol/l), and higher in epithelioid type (n=13; median 15.4, range 2.2–82.8 nmol/l) than in sarcomatoid (n=4; median 13.8, range 2.85–10.45 nmol/l), though there were no significant differences (P=0.158 and 0.389, respectively).
SMRP and asbestos exposure
Occupational asbestos exposure was revealed in 21 patients with MPM. We examined the duration of asbestos exposure and the SMRP value in the pleural fluid, but no correlation was shown (PCI, −0.069). Quantification of asbestos bodies was performed in 17 cases of MPM. The median number of bodies was 2,180 (239–526,000) per gram of dried lung. We examined the correlation between the SMRP value in pleural fluid and the number of asbestos bodies, but no correlation was found (PCI, −0.156). Survival data was available in 22 cases. No correlation was found between the SMRP value and survival (PCI, −0.179). We compared the survival of two groups, those with a lower concentration of SMRP (≤8.0 nmol/l) and those with a higher concentration, but no statistical difference was demonstrated (data not shown).
SMRP value for dif ferential diagnosis
The median concentration of SMRP in MPM, LC, BAP, TP and CHF were 11.5 (range 0.9–82.8), 5.2 (0.05–36.4), 6.65 (1.45–11.25), 3.20 (1.65–6.5) and 2.03 (1.35–2.8) nmol/l, respectively. The SMRP concentration was significantly higher in MPM than in the other diseases (P=0.001, Kruskal-Wallis test, Fig. 1). The area under the ROC curve (AUC) values of the MPM diagnosis was 0.75 [95% confidence interval (CI), 0.615–0.884] for the differential diagnosis from the other groups. Based on the cut-off value of 8 nmol/l, the sensitivity and specificity for diagnosis of MPM were 70.0 and 68.4%, respectively. The SMRP concentration in MPM was significantly higher than that in LC (P=0.004, Mann-Whitney U test). The AUC for the differential diagnosis of MPM and LC was 0.724 (95% CI, 0.583–0.866). Based on the cut-off value of 8 nmol/l, the sensitivity and specificity for diagnosis of MPM were 69.6 and 68.4%, respectively. The SMRP concentration in MPM was significantly higher than in BAP (P=0.004, Mann-Whitney U test). The AUC value for the differential diagnosis of MPM and BAP was 0.74 (95% CI, 0.586–0.894). Based on the cut-off value of 8 nmol/l, the sensitivity and specificity for diagnosis of MPM were 69.6 and 69.2%, respectively.
Figure 1.
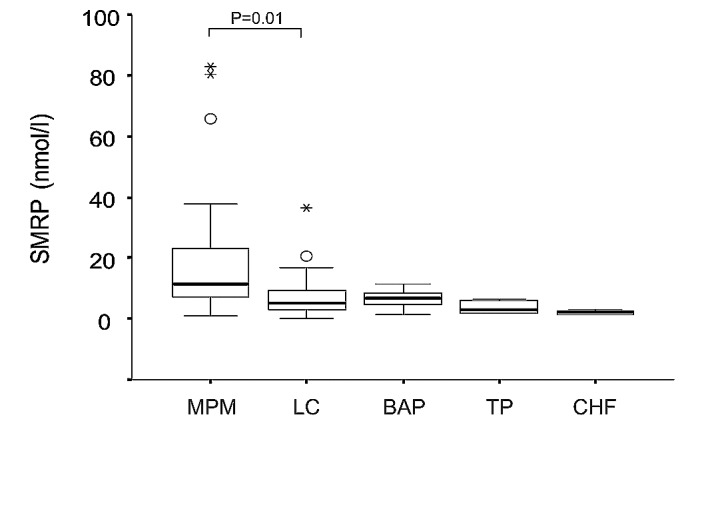
SMRP concentrations in pleural fluid. MPM, malignant pleural mesothelioma; LC, lung cancer; BAP, benign asbestos pleurisy; TP, tuberculosis pleurisy; CHF, chronic heart failure.
Discussion
In this study, we first examined the SMRP value in pleural fluid from patients with MPM. SMRP was higher in the epithelioid subtype than in the sarcomatoid, and higher in advanced stages (III and IV) than in early stages (I and II), though the differences were not statistically significant. These findings collaborate a previous study by Scherpereel et al (21). They examined the SMRP values, both in serum and pleural fluid, and reported that SMRP both in serum and pleural fluid was higher in the epithelioid subtype and in advanced diseases of MPM. The differences in our study were not statistically significant, probably due to the small number of samples, but our results reflect a similar trend in MPM in Japan. In addition, we examined the correlation between pleural SMRP and overall survival of patients with MPM, but no correlation was found. The role of serum SMRP as a prognostic marker was examined by Cristaudo et al. In their study, a high SMRP level in serum was an independent negative prognostic factor in patients with MPM (19). The present study is the first report to examine the role of pleural SMRP as a prognostic factor, but these results should be interpreted carefully because of the small number of cases. Further studies are warranted to clarify the role of pleural SMRP as a prognosis predictive marker.
We next examined the usefulness of pleural SMRP as a diagnostic marker of MPM. We compared the SMRP value in the pleural fluid of MPM to that of LC, BAP, TP and CHF. The SMRP value in MPM was significantly higher than in the other diseases. Similar findings were also reported by Scherpereel et al (21). They reported that the serum or pleural fluid SMRP level was significantly higher in patients with MPM than in subjects with benign pleural lesions related to asbestos exposure (BPLAE) or in LC. In their report, BPLAE was defined based on the definition by the American Thoracic Society (27), which corresponds with BPE in our study. In our study, subjects with TP and CHF were also included as controls. TP is the single most frequent cause of death by an infectious agent and is also a major cause of pleural effusion (28). Several molecular markers in pleural effusion have been examined as diagnostic markers of TP (29), but the differential diagnosis is still often problematic in clinical practice. Our results revealed, for the first time, the usefulness of pleural SMRP to distinguish MPM and TP.
We also analyzed the correlations between the SMRP concentration and asbestos exposure. We determined the number of asbestos bodies in the lungs of patients with MPM. The duration of occupational asbestos exposure was determined through patient interview. As a result, no correlation was revealed between SMRP values and the duration of asbestos exposure or asbestos bodies in the lung. These findings indicate that elevation of SMRP in the pleural effusion of MPM is not influenced by asbestos, but is one of the cancer-specific events. The mechanisms of accumulation of SMRP in pleural fluid have not as yet been established. SMRP is reported as a proteolytically cleaved fragment of membrane-bound mesothelin (17). The release of SMRP could also be due to a frameshift mutation of the protein (21). Further studies are warranted to examine the mechanisms involved in the elevation of SMRP in MPM.
In conclusion, we examined the SMRP concentration in pleural fluid from patients with MPM, LC, BAP, TP and CHF and demonstrated that the SMRP value in MPM was significantly higher than that in the other diseases. These results indicate the usefulness of pleural SMRP as a diagnostic marker of MPM.
Acknowledgments
This research is a part of the research and development, and dissemination projects related to the 13 fields of occupational injuries and illnesses of the Japan Labour Health and Welfare Organization. This research is supported by the Program for the Promotion of Fundamental Studies in Health Science of the National Institute of Biomedical Innovation of Japan.
References
- 1.Ohshima H. Five cases with mesothelioma living near a now-defunct asbestos cement plant in Amagasaki city. Mainichi Newspaper (in Japanese) 2005 Jun 29;:1. [Google Scholar]
- 2.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 3.Ray M, Kindler HL. Malignant pleural mesothelioma: an update on biomarkers and treatment. Chest. 2009;136:888–896. doi: 10.1378/chest.08-2665. [DOI] [PubMed] [Google Scholar]
- 4.Shi HZ, Liang QL, Jiang J, Qin XJ, Yang HB. Diagnostic value of carcinoembryonic antigen in malignant pleural effusion: a meta-analysis. Respirology. 2008;13:518–527. doi: 10.1111/j.1440-1843.2008.01291.x. [DOI] [PubMed] [Google Scholar]
- 5.Liang QL, Shi HZ, Qin XJ, Liang XD, Jiang J, Yang HB. Diagnostic accuracy of tumour markers for malignant pleural effusion: a meta-analysis. Thorax. 2008;63:35–41. doi: 10.1136/thx.2007.077958. [DOI] [PubMed] [Google Scholar]
- 6.Aoe K, Hiraki A, Maeda T, et al. Soluble receptor-binding cancer antigen expressed on SiSo cells in pleural fluid: a potential diagnostic marker for malignant pleural effusion. Chest. 2004;126:1195–1197. doi: 10.1378/chest.126.4.1195. [DOI] [PubMed] [Google Scholar]
- 7.Rump A, Morikawa Y, Tanaka M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 8.Chang K, Pai LH, Pass H, et al. Monoclonal antibody K1 reacts with epithelial mesothelioma but not with lung adenocarcinoma. Am J Surg Pathol. 1992;16:259–268. doi: 10.1097/00000478-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–197. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- 11.Ordonez NG. The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol. 2003;27:1031–1051. doi: 10.1097/00000478-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Scholler N, Fu N, Yang Y, et al. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci USA. 1999;96:11531–11536. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frierson HF, Jr, Moskaluk CA, Powell SM, et al. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum Pathol. 2003;34:605–609. doi: 10.1016/s0046-8177(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharjee A, Richards WG, Staunton J, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci USA. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornick JL, Lauwers GY, Odze RD. Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver. Am J Surg Pathol. 2005;29:381–389. doi: 10.1097/01.pas.0000149710.01559.fe. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe H, Okada G, Ohtsubo K, et al. Expression of mesothelin mRNA in pure pancreatic juice from patients with pancreatic carcinoma, intraductal papillary mucinous neoplasm of the pancreas and chronic pancreatitis. Pancreas. 2005;30:349–354. doi: 10.1097/01.mpa.0000160281.56828.76. [DOI] [PubMed] [Google Scholar]
- 17.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 18.Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 19.Cristaudo A, Foddis R, Vivaldi A, et al. Clinical significance of serum mesothelin in patients with mesothelioma and lung cancer. Clin Cancer Res. 2007;13:5076–5081. doi: 10.1158/1078-0432.CCR-07-0629. [DOI] [PubMed] [Google Scholar]
- 20.Schneider J, Hoffmann H, Dienemann H, Herth FJ, Meister M, Muley T. Diagnostic and prognostic value of soluble mesothelin-related proteins in patients with malignant pleural mesothelioma in comparison with benign asbestosis and lung cancer. J Thorac Oncol. 2008;3:1317–1324. doi: 10.1097/JTO.0b013e318187491c. [DOI] [PubMed] [Google Scholar]
- 21.Scherpereel A, Grigoriu B, Conti M, et al. Soluble mesothelin-related peptides in the diagnosis of malignant pleural mesothelioma. Am J Respir Crit Care Med. 2006;173:1155–1160. doi: 10.1164/rccm.200511-1789OC. [DOI] [PubMed] [Google Scholar]
- 22.Pass HI, Wali A, Tang N, et al. Soluble mesothelin-related peptide level elevation in mesothelioma serum and pleural effusions. Ann Thorac Surg. 2008;85:265–272. doi: 10.1016/j.athoracsur.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 23.Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma from the International Mesothelioma Interest Group. Lung Cancer. 1996;14:1–12. doi: 10.1016/0169-5002(95)00508-0. [DOI] [PubMed] [Google Scholar]
- 24.Histological Typing of Lung and Pleural Tumors. World Health Organization; Geneva: 1999. [Google Scholar]
- 25.Mountain CF. Revisions in the International System for staging lung cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 26.Kohyama N, Suzuki Y. Analysis of asbestos fibers in lung parenchyma, pleural plaques and mesothelioma tissues of North American insulation workers. Ann NY Acad Sci. 1991;643:27–52. doi: 10.1111/j.1749-6632.1991.tb24442.x. [DOI] [PubMed] [Google Scholar]
- 27.Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med. 2004;170:691–715. doi: 10.1164/rccm.200310-1436ST. [DOI] [PubMed] [Google Scholar]
- 28.Raviglione MC, Luelmo F. Update on the global epidemiology of tuberculosis. Curr Issues Public Health. 1996;2:192–197. [PubMed] [Google Scholar]
- 29.Hiraki A, Aoe K, Eda R, et al. Comparison of six biological markers for the diagnosis of tuberculous pleuritis. Chest. 2004;125:987–989. doi: 10.1378/chest.125.3.987. [DOI] [PubMed] [Google Scholar]


