Abstract
Background
Triple X syndrome is a sex chromosomal aneuploidy condition characterized by tall stature, microcephaly, hypertelorism, congenital abnormalities, and motor and language delays. It is mainly derived from maternal nondisjunctional errors during meiosis. To highlight the clinical features and diagnosis of triple X syndrome, we present a rare phenotype of the syndrome.
Case Presentation
A 5.9 year-old girl was admitted to our hospital because of short stature. Both her height and weight were below the 3rd percentile compared to the normal peers. She was found with mild motor and speech delay. Laboratory investigation showed low level of IGF-1 and zinc, elevated estradiol level and normal result of arginine provocation test.
Conclusion
Our data suggest that triple X syndrome should also be suspected in patients with short stature, elevated estradiol and low level of IGF-1, even with normal result of arginine provocation test.
Keywords: 47, XXX; Insulin-Like Growth Factor-1; Sex Chromosome Aneuploidy; Short Stature; Triple X Syndrome
Introduction
Triple X syndrome (trisomy X, 47,XXX), first described by Jacobs 1959, is a sex chromosomal aneuploidy condition with female phenotype[1]. The incidence of the syndrome is estimated at 10.7 per 100000 live born girls[2]. Its symptoms vary very widely, including tall stature, hypertelorism, epicanthal folds, clinodactyly, congenital heart disease, genitourinary and some other anomalies,[3]. Neuroimaging studies showed significantly smaller brain volume in these patients[4], which might associate with poor learning and language skills at school age[5]. These could eventually lead to shyness, stress, and disturbance in their interpersonal relationships. Some cases even may experience delayed menarche and premature ovarian function. Mortality of the syndrome is significantly higher than in age-matched females[2].
Herein, we report a case of 47,XXX who was discovered to be slow growing. To our knowledge, this is the first case report of a triple-X karyotype with short stature. The patient's parents provided written informed consent for karyotype sampling and taking pictures.
Case Presentation
A 5.9-year-old girl presented to our unit due to slow growing for 4 years. She was the only child of unrelated parents. She was born at term by uterine-incision delivery following an uneventful pregnancy, weighing 2700 g and measuring 47 cm. No medication history was reported. She started walking independently and speaking at the 17th month. Her mother was 27 years and father was 32 years at conception, without any history of disease or use of drugs before or during pregnancy. Her mother and father had a height of 154 cm and 164 cm, respectively. Both of them are healthy and have normal karyotypes. Her family history revealed that no clinical features were noted in other family members, and they had not examined their karyotype.
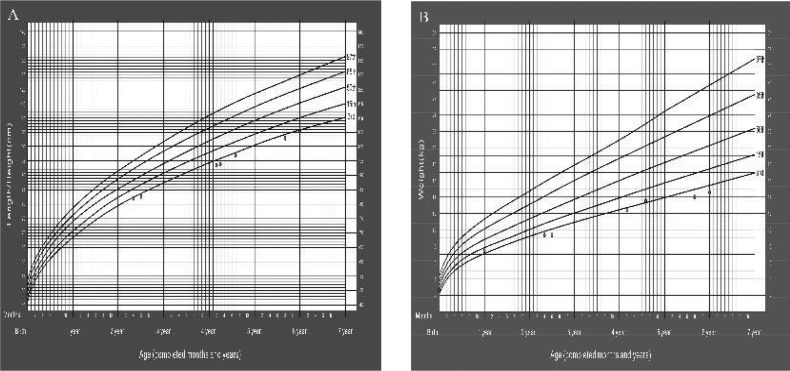
The girl was 105 cm (<3rd percentile for normal population, height SD score -1.9) with a weight of 14.6 kg (<3rd percentile for normal population, weight SD score <-2SD). Her body mass index (BMI) was 13.2 (<15th percentile for normal population, BMI SD score <-1SD) and head circumference 49.5 cm (percentile for normal population, BMI SD score). She grew 4-5 cm every year in the past 4 years, as shown in Fig. 1.
Fig. 1.
The growth of height (A) and weight (B) from birth to 5.9 years. The patient was below the 3rd percentile according to the WHO Child Growth Standards.
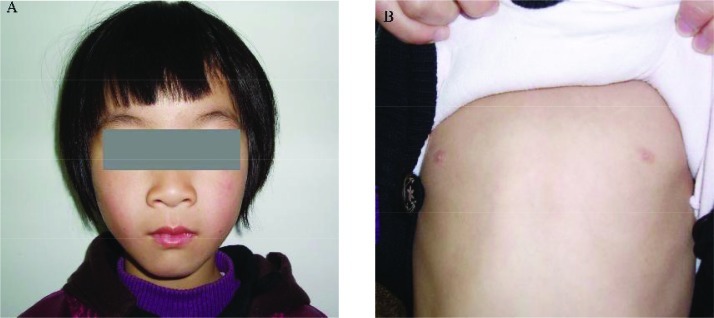
She had a higher interpupillary distance with Tanner 1 stage of breasts (Fig. 2a-b). The genitalia were of normal female phenotype. No other abnormal features were noted.
Fig. 2.
A: Normal features in appearance. B: widened papillae distance
The score on the Peabody Picture Vocabulary Test and the social viability measuring list on infants-junior middle school students revised by Zuo Qihua[6] were within the average range. The outcome of Gesell Developmental Schedules indicated mild development delay in gross motor, fine motor and language (Table 1).
Table 1.
Development quotient (DQ) scores and developmental age (DA) of Gesell
| DQ | DA (month) | |
|---|---|---|
| Adaptive area | 78 | 56.1 |
| Gross Motor | 64 | 46 |
| Fine Motor | 75 | 54 |
| Language | 73 | 52.7 |
| Social interaction | 82 | 58.8 |
Normal ≥ 85; borderline development 75 < DQ < 85; mild delay 55 ≤ DQ ≤ 75; moderate delay 40 ≤ DQ ≤ 54; severe delay 25 ≤ DQ ≤ 39; extremely severe delay 25 < DQ
Laboratory tests showed decreased serum insulin-like growth factor-1 (IGF-1) from 46.4 mg/L (normal range 42-114 mg/L) measured 2 years ago to 31.1 mg/L (normal range 60-158 mg/L). The peak value of growth hormone (GH) in arginine provocation test was 11.4 mg/L, while 36.6 mg/L in insulin provocation test. Both of them were in normal range. Her blood zinc level was normal two years ago, but this time, it was 69.4 µg/L (normal range 72-180 µg/L). Basal hormone test 2 years ago indicated follicle stimulating hormone (FSH 18.6 IU/L; normal range 0.5-3.7 IU/L), luteinizing hormone (LH 2.3 IU/L; normal range 0.6∼1.7 mIU/mL) and prolactin (PRL 29.00 IU/L; normal range 2.34-26.7 IU/L) were elevated, while this time the basal level of LH was <0.10 IU/L and estradiol was 25.8 ng/L (normal range <20 ng/L). Other parameters including liver and kidney function, blood routine test, blood glucose, cortisol and adrenocorticotropic hormone, thyroid function, insulin, and hepatitis virus were all within normal limits.
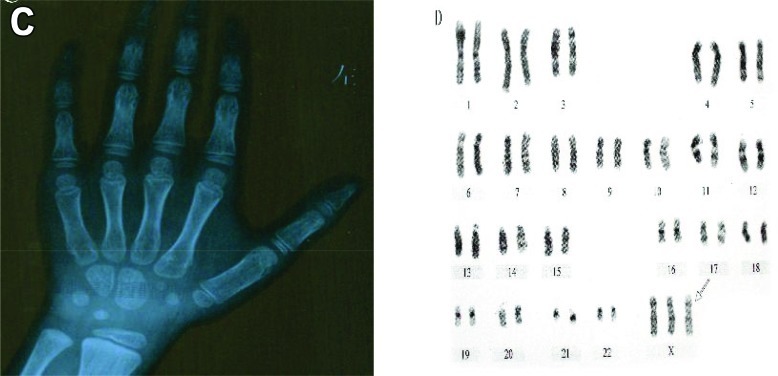
Bone radiographic imaging examination of the left carpal demonstrated that there was 7/10 wrist ossification center, the difference between bone age and chronological age was in normal range (Fig. 3A). Chromosome analysis using G-banding technique demonstrated a 47,XXX karyotype (Fig. 3B). The ultrasound examination of heart, uterus, ovary and urinary system, as well as cranial magnetic resonance imaging (MRI) were normal.
Fig. 3.
A, Radiological findings of bone age. B, 47, XXX Karyotype of the patient
Discussion
Triple X syndrome is usually of sporadic origin. X chromosomes in these patients fail to separate during cell division, in a process called nondisjunction. It mostly derives from maternal nondisjunctional errors during meiosisⅠ(63%) or Ⅱ(17.4%). Only one of three X chromosomes is activated and the other two are inactivated to Barr bodies. The variable phenotypic abnormalities mentioned above are thought to be related to the over expression of the genes situated on the extra X chromosomes that escape X-inactivation [3]. Advanced maternal age and aberrant recombination are risk factors of the syndrome [7].
It is reported that only 10% of the patients were diagnosed [3], because the identification of a fetus with 47,XXX on ultrasound is difficult and prenatal diagnosis via amniocentesis, chorionic villi sampling or postnatal karyotype analysis are not routine in the clinic. Variable symptoms will also contribute to the high rate of misdiagnosis. In this case, other family members were phenotypically normal and the girl would not have been suspected from physical examination. Thus, the karyotype of 47,XXX was an unexpected finding.
Triple X females are tending to display moderately tall stature [8], their final height ranges from -1 to 3 SDS of the normal population. It was supposed to be related to the short-stature-homeobox-containing gene (SHOX gene) in the pseudoautosomal region of X and Y chromosomes [9]. The excessive copies of the gene will prolong the period of growth, while haploinsufficiecy of it may lead to short stature. Also, the alteration of non-inactivated region and hormone factors might contribute to the height increase [10]. In this report, the girl was short. To our knowledge, this is the first case of a triple X patient with short stature. Her body length ranged between the 15th to 50th percentile in the first year, and later on, it was below the 3rd percentile, but she grew about 4∼5cm annually in recent years. So, in our case, the height of the patient was opposite to previous reports in the literature. There was no evidence showing that her short stature was related to growth hormone deficiency, because the result of the arginine provocation test was normal. The low level of blood zinc and IGF-1 might have an effect on her stature in some extent, since low blood zinc level will reduce the level of IGF-1 and lead to slow growing. However, the blood zinc and IGF-1 level were in normal ranges 2 years ago, but they were significantly decreased this time, especially the IGF-1 level, which was below -2SD for age. Meanwhile, we found that the girl's growth rate did not change a lot in the past two years. Thus, it may indicate that the circulating levels of IGF-1 and blood zinc level are not the main causes of the abnormal growth pattern in our case. This was the same as Lise Aksglaede's opinion that growth pattern was not reflected in circulating levels of IFG-1 in trisomy[11]. Some studies proposed that neither SHOX overdosage nor estrogen deficiency alone were sufficient to lead to a tall stature, since their combination permitted a prolonged growth period and a higher final height[9]. In this study, the girl showed elevated estrogen level. Thus, the SHOX over dosage per se may not lead to over growth and we hypothesized that a mutation in the SHOX gene may result in a short height in our case. But we did not do the FISH analysis of SHOX, so we cannot make any conclusions.
Previous literature showed that 47,XXX females usuaLy had high levels of estrogen and progesterone, causing menstrual disorder and sexual precocity [12]. However, most of them will have normal reproductive functions. In our case, when the girl was 4.2 years old, her hormone test showed that the level of FSH, LH and PRL were all above the normal range, especially FSH. This does not happen before puberty in normal populations. Thus, it indicates that in our case the hypothalamus-adenohypophysis-gonadal (HPG) axis was activated in advance. The level of estradiol increased two years later, while the level of FSH and LH decreased to normal and below the lower limit, respectively. It prevented the estradiol from increasing too much, and no physical changes occurred. The abnormal level of gonadal hormone in our case was probably due to the existence of the extra X chromosome and the expression of genes which had escaped X-inactivation. However, continued monitoring into adolescence will be required for evaluation of hormone levels and pubertal development.
Some studies reported that 50% of 47,XXX females have delayed motor development and poor language skills[5]. In our case, 17 months of age was the time first words were pronounced and first steps taken, showing a slight delay from the normal population. The result of Gesell developmental schedule demonstrated minor development delay on motor and language skills. These characteristics were consistent with the clinical features in previous reports.
Conclusion
Our case provides a rare example of 47,XXX associated with short stature, elevated level of estradiol and decreased IGF-1 level. Therefore, clinicians should be aware of possible association between triple X and short stature. Further study on the correlation and mechanism between 47,XXX and abnormal growth is required.
Acknowledgments
We thank parents of the patient for permitting to publish her data. This work was supported by grants from the Zhejiang Health Bureau Fund (2006QN017).
References
- 1.Jacobs PA, Baikie AG, Brown WM, et al. Evidence for the existence of the human “super female”. Lancet. 1959;2(7100):423–5. doi: 10.1016/s0140-6736(59)90415-5. [DOI] [PubMed] [Google Scholar]
- 2.Stochholm K, Juul S, Gravholt CH. Mortality and incidence in women with 47,XXX and variants. Am J Med Genet A. 2010;152A(2):367–72. doi: 10.1002/ajmg.a.33214. [DOI] [PubMed] [Google Scholar]
- 3.Tartaglia NR, HoweL S, Sutherland A, et al. A review of trisomy X (47,XXX) Orphanet J Rare Dis. 2010;5:8. doi: 10.1186/1750-1172-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenroot RK, Lee NR, Giedd JN. Effects of sex chromosome aneuploidies on brain development: evidence from neuroimaging studies. Dev Disabil Res Rev. 2009;15(4):318–27. doi: 10.1002/ddrr.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tennes K, Puck M, Bryant K, et al. A develop-mental study of girls with trisomy X. Am J Hum Genet. 1975;27(1):71–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo Qi-hua, Zhang Zhi-xiang, Wei-lan L. Infants-Junior Middle School Students' Social-Life Abilities Scales. Beijing: Beijing Medical University; 1988. pp. 1–68. [Google Scholar]
- 7.Reish O, Berryman T, Cunningham TR, et al. Reduced recombination in maternal meiosis coupled with non-disjunction at meiosis II leading to recurrent 47,XXX. Chromosome Res. 2004;12(2):125–32. doi: 10.1023/b:chro.0000013164.56757.bd. [DOI] [PubMed] [Google Scholar]
- 8.Ottesen AM, Aksglaede L, Garn I, et al. Increased number of sex chromosomes affects height in a nonlinear fashion: a study of 305 patients with sex chromosome aneuploidy. Am J Med Genet A. 2010;152A(5):1206–12. doi: 10.1002/ajmg.a.33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamson KA, Cross I, Batch JA, et al. Trisomy of the short stature homeobox-containing gene (SHOX), resulting from a duplication-deletion of the X chromosome. Clin Endocrinol (Oxf) 2002;56(5):671–5. doi: 10.1046/j.1365-2265.2002.01504.x. [DOI] [PubMed] [Google Scholar]
- 10.Ogata T, Matsuo N. Sex chromosome aberrations and stature: deduction of the principal factors involved in the determination of adult height. Hum Genet. 1993;91(6):551–62. doi: 10.1007/BF00205079. [DOI] [PubMed] [Google Scholar]
- 11.Aksglaede L, Skakkebaek NE, Juul A. Abnormal sex chromosome constitution and longitudinal growth: serum levels of insulin-like growth factor (IGF)-I, IGF binding protein-3, luteinizing hormone, and testosterone in 109 males with 47,XXY, 47,XYY, or sex-determining region of the Y chromosome (SRY)-positive 46,XX karyotypes. J Clin Endocrinol Metab. 2008;93(1):169–76. doi: 10.1210/jc.2007-1426. [DOI] [PubMed] [Google Scholar]
- 12.Goswami R, Goswami D, Kabra M, et al. Prevalence of the triple X syndrome in phenotypically normal women with premature ovarian failure and its association with autoimmune thyroid disorders. Fertil Steril. 2003;80(4):1052–4. doi: 10.1016/s0015-0282(03)01121-x. [DOI] [PubMed] [Google Scholar]