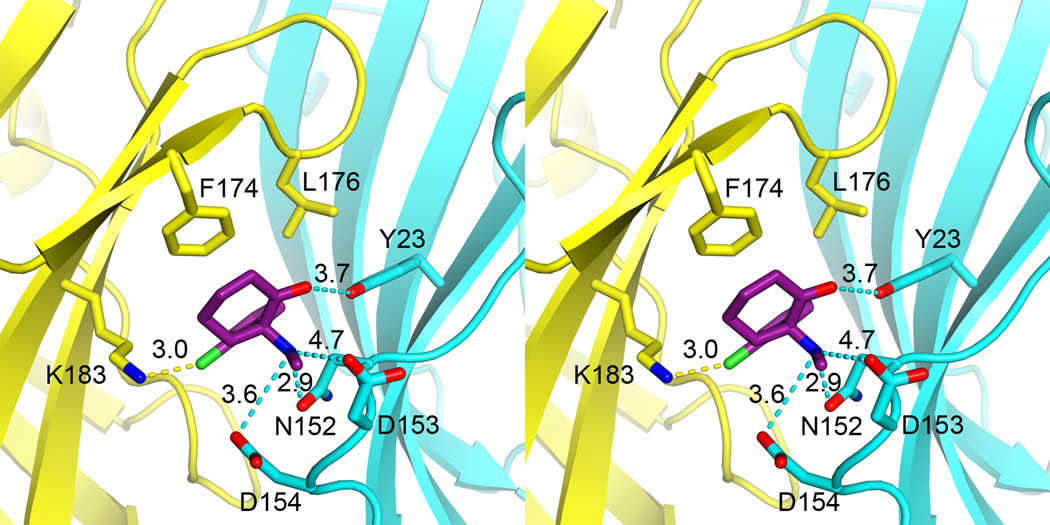
Figure 3. Stereo view of atomic details of the ketamine binding pocket.
Dashed lines indicate distances in Å between the ketamine atoms and the relating residues for electrostatic interactions. Residues on the principal and complementary sites of the pocket are colored in yellow and cyan, respectively. Note the position of ketamine relative to N152, in which the mutation affected GLIC activation and ketamine inhibition. See also Figures S3 and S5, and Table S1.