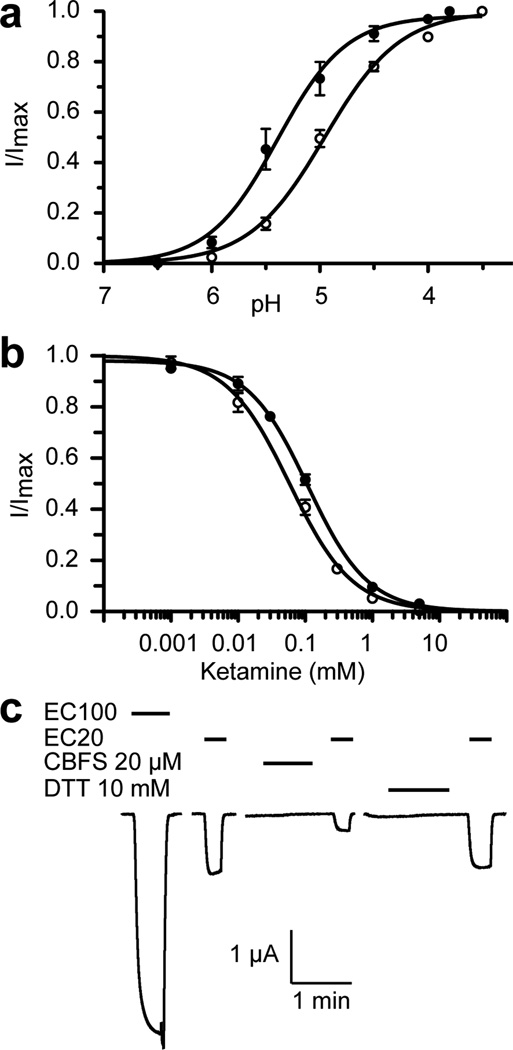
Figure 4. Functional relevance of the ketamine-binding site.
(a) A single mutation of N152C in the binding pocket altered pH activation of the channel. Currents of the N152C mutant (solid circle) and the wide type GLIC (open circle) expressed in Xenopus oocytes were normalized to the maximum current at pH 3.8 or 3.5, respectively. (b) The N152C mutation weakened the ketamine inhibition. Responses of the N152 mutant (solid circle) and the wide type GLIC (open circle) are expressed as the fraction of current induced at EC20 in the presence of the indicated concentrations of ketamine relative to that in the absence of ketamine. (c) Representative current traces of the N152C_C27A mutant at different pH values, before and after labeling of 8-(chloromercuri)-2-dibenzofuransulfonic acid (CBFS) at a concentration of 20 µM, and after the treatment of 10-mM dithiothreitol (DTT). The application of CBFS or DTT to Xenopus oocytes lasted for ~2 minutes and was followed by the subsequent application of pH 7.4 buffer before a new measurement. The data in (a) and (b) are reported as the mean ± SEM from n ≥ 8 oocytes and fit to the Hill equation. Error bars less than the symbol size are not visible. See also Figure S4.