Figure 3.
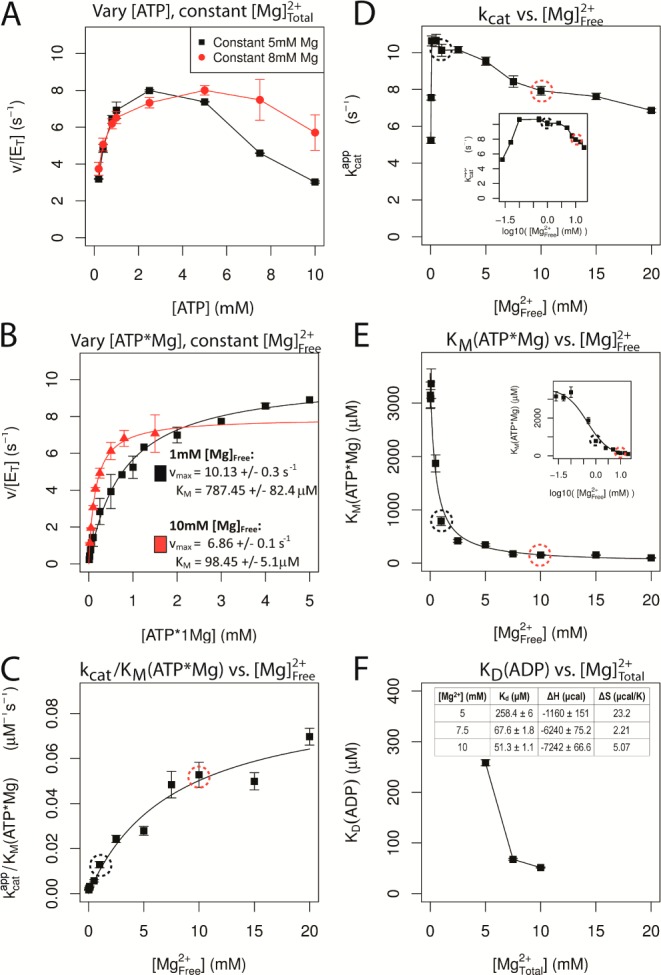
Cooperativity between nucleotide and Mg2+ binding to CDK2. (A) Dependence of the multiple turnover kinase reaction on the concentration of ATP at constant [Mg2+]total. Histone substrate was saturating and kinase activity was measured using a coupled assay (see Experimental Methods for details). Concentration of total Mg2+ was fixed at either 5 or 8 mM. (B) Multiple turnover kinetics in which the concentration of ATP·Mg2+ was varied at a fixed concentration of either 1 (black) or 10 mM (red) free Mg2+. Velocity was fit by the Michaelis–Menten equation (lines). (C–E) Resulting steady state constants from these experiments and from data collected at additional concentrations of free Mg2+ (see Supporting Information Figures 4–14). (C) Value of kcat/KMATP·Mg was fit to a hyperbolic binding equation, indicating a very weak affinity of ∼10 mM for Mg2+ binding to free enzyme in the absence of nucleotide. (D) Value of kcat shows a complex dependence on the concentration of free Mg2+ with a highly cooperative stimulation at very low Mg2+ and a modest decrease in kcat between 1 and 20 mM Mg2+. Inset: log10 scale. (E) Value of KM for ATP·Mg2+ is dramatically decreased by binding of a second Mg2+ ion. (F) Dissociation constant for ADP was determined by isothermal calorimetry as a function of total Mg2+ (see Supporting Information for details).
