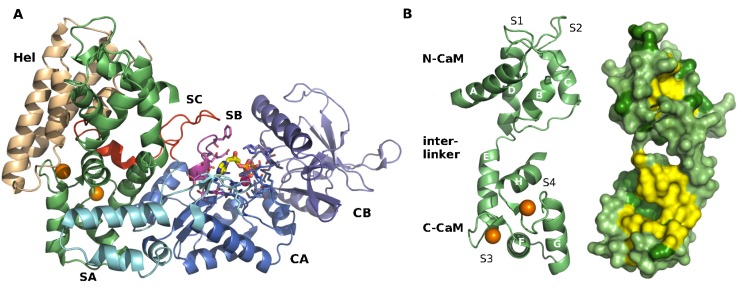
Figure 1.
X-ray crystallographic structure of the EF-CaM complex [23]. (A) EF and CaM are displayed in cartoon representations. Calmodulin (CaM) is in green lime and loaded with two calcium ions (spheres in orange). The helical domain Hel of EF is in wheat, the CA and CB domains that form the catalytic core of EF are in marine and purple respectively. The three switches SA, SB and SC are colored in cyan, magenta and red. The catalytic residues and the ligand 3'-deoxy ATP are drawn in sticks and the Yb3+ ion is drawn as a magenta sphere; (B) Cartoon and surface representations of CaM structure are displayed from different views. On the left, the eight helices of CaM are labeled: A, B, and C in N-CaM; D and E in the interlinker; and F, G, and H in C-CaM. Calcium-binding loops S1 and S2 in N-CaM, S3 and S4 in C-CaM are indicated. Two Ca2+ ions are bound to S3 and S4 (spheres in orange). On the right, CaM hydrophobic patches as defined by Yang et al. [30] are colored in yellow and the other hydrophobic residues are colored in forest green.