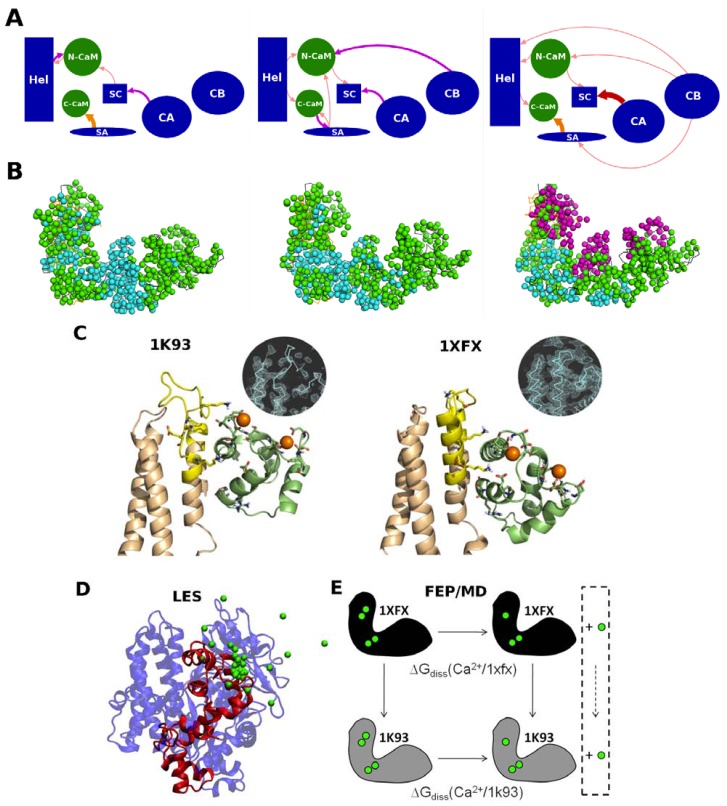
Figure 3.
Calcium effect on the EF-CaM complex.Energetical influences and dynamical correlations computed in the EF-CaM complex. (A) the energetical influences between the different domains of EF (in blue) and CaM (in green) are drawn as arrows whose color and thickness indicate the intensities of the influences, as seen by MMPBSA energy dependency maps [49]; (B) the residues of the EF-CaM complex are represented by their C-α atoms and colored in cyan when their dynamical correlations are low (<0.5), in green when their correlations are mean (0.5–0.6) and in magenta when their correlations are high (>0.6). In the 2 Ca2+ bound complex (in the middle), the energetical influences are located in the vicinity of the EF/CaM interface and the most residue are correlated. Removal of calcium (on the left) reduces the energetical influences and a region of uncorrelated residues appears at the interface between the two proteins. Upon calcium addition (on the right), new energetical influences are observed even at long distances, and three sets of highly correlated residues were observed; (C) Interface between the Hel domain of EF and N-CaM in the EF-CaM complex. The conformations taken after 8 ns of MD simulations starting from 1K93 (on the left, chains C and F) and 1XFX (on the right, chains A and O) are represented in cartoon. EF Hel domain is colored in wheat with the inter L–M loop highlighted in yellow. N-CaM is colored in green lime. Residues involved in the EF/CaM interaction and residues forming CaM calcium binding sites S1 and S2 are drawn in sticks. Calcium ions are displayed as orange spheres. The inserts represent the electron density maps of the starting crystallographic structures, contoured at 1.10 s; (D) Principles of locally enhanced sampling (LES) methods; a snapshot from the LES simulations (protein in cartoons, ions in green spheres): to enhance the probability of calcium dissociation events, one Ca2+ ion is replicated in multiple copies; (E) Principles of free energy perturbation (FEP). The thermodynamic cycle used in the FEP method to compare calcium dissociation free energies between two protein conformational states (1k93-4Ca and 1xfx-4Ca complexes).