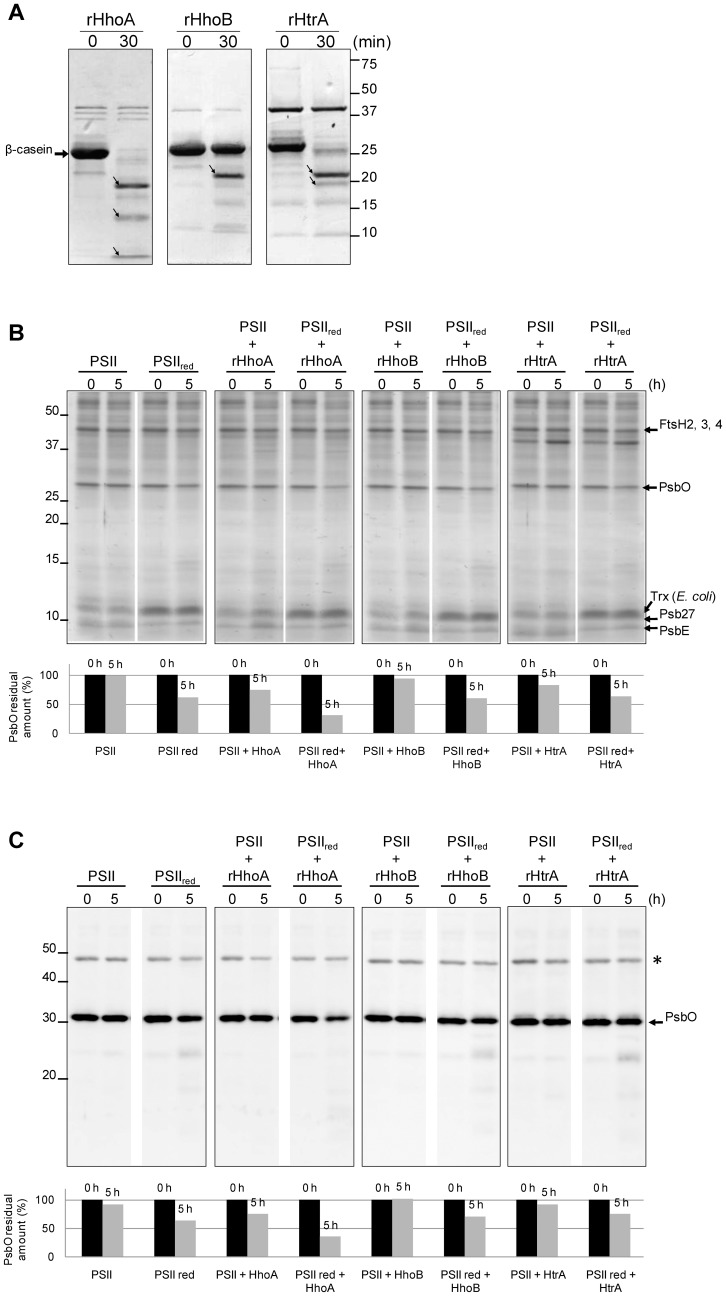
Figure 1. Cyanobacterial PsbO is degraded by rHhoA in a redox-dependent manner.
(A) Proteolytic activity of recombinant Synechocystis 6803 Deg/HtrA proteases against naturally unfolded β-casein. Small arrows indicate β-casein degradation fragments. (B) A PSII-enriched fraction was isolated from Synechocystis 6803 using the HT3 mutant with His-tagged CP47. The PSII-enriched fraction was incubated for 5 h in the absence (PSII) or the presence (PSIIred) of the thioredoxin system, either with no protease (panel 1) or with rHhoA (panel 2), rHhoB (panel 3), or rHtrA (panel 4). The proteins (15 µg) were separated by SDS-PAGE and analyzed with CBB staining. Identity of the named bands was confirmed using mass spectrometry. (C) After SDS-PAGE proteins were transferred to PVDF membranes and immunostained using an antibody directed against PsbO. Bars below the gels and blots show the integrated density values of the corresponding bands as quantified by Image J software. The asterisk marks an nonspecific cross-reacting band.