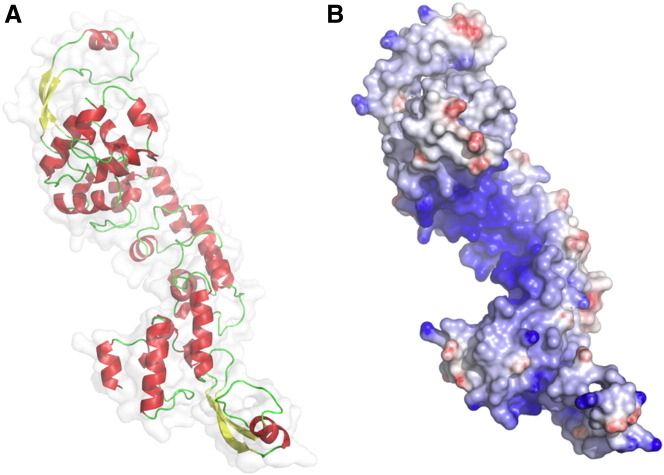
Fig. 4.
Homology modeling of D-MTERF5. (A) Overall structure of D-MTERF5 (residues 62–443 of the mature protein) obtained by comparison with human mTERF, by using the Swiss-Model Automated Protein Modeling Server, in which predicted alpha-helices are in red and beta-sheets are in yellow. (B) Molecular surface of D-MTERF5 colored according to the electrostatic surface potential (blue + 12 kT, red − 12 kT).