Abstract
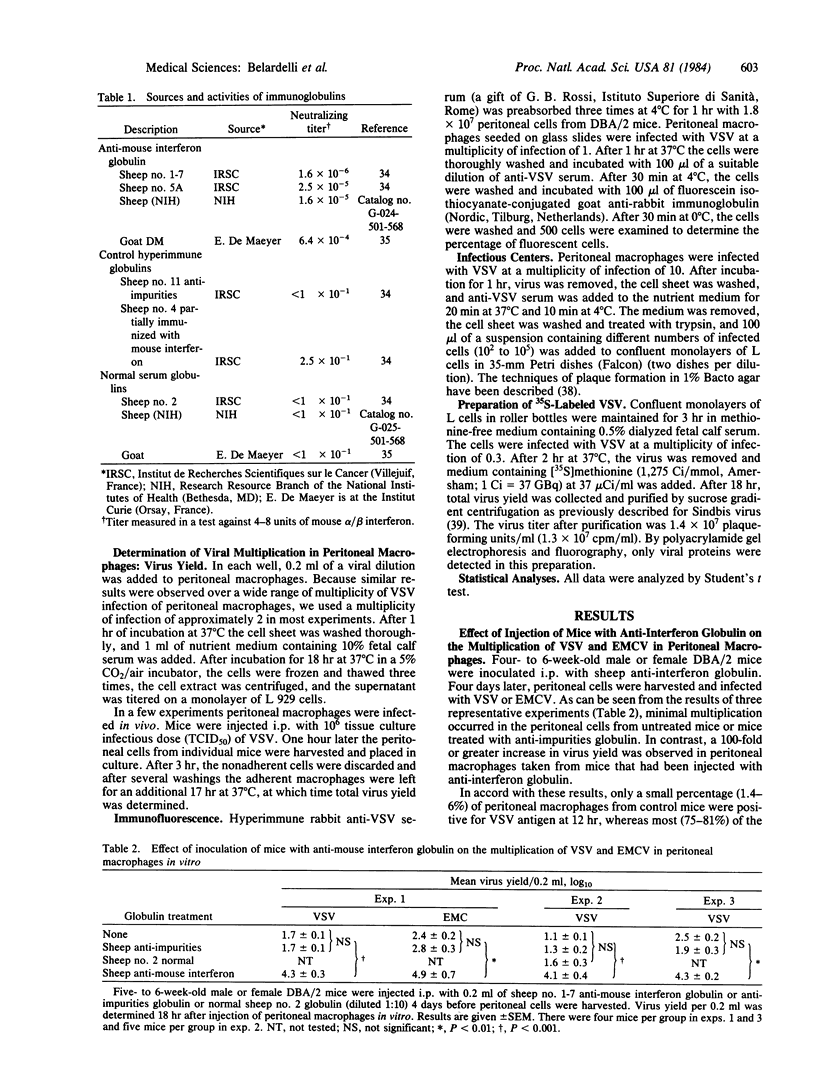
Vesicular stomatitis virus (VSV) and encephalomyocarditis virus (EMCV) multiply in only a small percentage of peritoneal macrophages freshly explanted from 4- to 6-week-old male or female DBA/2, BALB/c, C3H, C57BL/6, or Swiss mice. However, when these mice were injected intraperitoneally with potent sheep (or goat) anti-mouse interferon alpha/beta globulin 4 days prior to harvesting peritoneal macrophages, the viruses multiplied to high titers and most of the cells were infected, as determined by total virus yield (VSV and EMCV), percentage of VSV antigen-positive cells (immunofluorescence), and determination of VSV infectious centers. This effect was not observed when mice were inoculated with other sheep hyperimmune or normal serum globulins. Anti-interferon globulin appeared to act in vivo because incubation of this globulin with peritoneal macrophages during the period of cell attachment or during the 18 hr after virus absorption did not render these cells permissive for VSV. Injection of mice with anti-interferon globulin did not affect the binding and uptake of labeled VSV by peritoneal macrophages. Although the underlying mechanism of this phenomenon is unknown, the results suggest that there may be low levels of endogenous interferon that contribute to host defense by maintaining some cells in an antiviral state.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C. On the role of mononuclear phagocytes in immunity against viruses. Prog Med Virol. 1974;18(0):15–31. [PubMed] [Google Scholar]
- Bang F. B. The use of a genetically incompatible combination of host and virus (MHV) for the study of mechanisms of host resistance. Adv Exp Med Biol. 1981;142:359–373. doi: 10.1007/978-1-4757-0456-3_30. [DOI] [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto A., Rossi G. B., Amici C., Belardelli F., Cioè L., Carruba G., Carrasco L. Inhibition of animal virus production by means of translation inhibitors unable to penetrate normal cells. Virology. 1980 Oct 15;106(1):123–132. doi: 10.1016/0042-6822(80)90227-5. [DOI] [PubMed] [Google Scholar]
- Brouty-Boyé D. High sensitivity of C3H mouse embryo-derived cell line to the antiviral activity of interferon. Proc Soc Exp Biol Med. 1977 Jul;155(3):438–439. doi: 10.3181/00379727-155-39824. [DOI] [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J. Delayed hypersensitivity to Newcastle disease virus in high and low interferon-producing mice. J Immunol. 1983 May;130(5):2392–2396. [PubMed] [Google Scholar]
- Dickinson L., Griffiths A. J. The pathogenesis of experimental infections with encephalomyocarditis (EMC) virus. Br J Exp Pathol. 1966 Feb;47(1):35–44. [PMC free article] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Macrophages as a cellular expression of inherited natural resistance. Proc Natl Acad Sci U S A. 1962 Feb;48:160–165. doi: 10.1073/pnas.48.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow L. A. Cellular immunity in host resistance to viral infections. Arch Intern Med. 1970 Jul;126(1):125–134. [PubMed] [Google Scholar]
- Gresser I., Bourali C., Thomas M. T., Falcoff E. Effect of repeated inoculation of interferon preparations on infection of mice with encephalomyocarditis virus. Proc Soc Exp Biol Med. 1968 Feb;127(2):491–496. doi: 10.3181/00379727-127-32723. [DOI] [PubMed] [Google Scholar]
- Gresser I., Lang D. J. Relationships between viruses and leucocytes. Prog Med Virol. 1966;8:62–130. [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Bandu M. E., Maury C., Brouty-Boyé D. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. I. Rapid evolution of encephalomyocarditis virus infection. J Exp Med. 1976 Nov 2;144(5):1305–1315. doi: 10.1084/jem.144.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Maury C., Bandu M. T. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. II. Studies with herpes simplex, Moloney sarcoma, vesicular stomatitis, Newcastle disease, and influenza viruses. J Exp Med. 1976 Nov 2;144(5):1316–1323. doi: 10.1084/jem.144.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O., Arnheiter H., Gresser I., Lindenmann J. Genetically determined, interferon-dependent resistance to influenza virus in mice. J Exp Med. 1979 Mar 1;149(3):601–612. doi: 10.1084/jem.149.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O., Arnheiter H., Lindenmann J. Natural, genetically determined resistance toward influenza virus in hemopoietic mouse chimeras. Role of mononuclear phagocytes. J Exp Med. 1979 Jul 1;150(1):117–126. doi: 10.1084/jem.150.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTOCH M., BANG F. B. Conversion of genetic resistance of mammalian cells to susceptibility to a virus infection. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1553–1559. doi: 10.1073/pnas.48.9.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTOCH M., WARWICK A., BANG F. B. The cellular nature of genetic susceptibility to a virus. J Exp Med. 1963 May 1;117:781–798. doi: 10.1084/jem.117.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSEL R. W., MONACO L., MARCHISIO M. A. THE SPECIFICITY OF THE CYTOTOXIC ACTION OF SILICA--A STUDY IN VITRO. Br J Exp Pathol. 1963 Aug;44:351–364. [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Engler H., Schröder C. H., Zawatzky R., Storch E. Herpes simplex virus type 1-induced interferon production and activation of natural killer cells in mice. J Gen Virol. 1983 Feb;64(Pt 2):437–441. doi: 10.1099/0022-1317-64-2-437. [DOI] [PubMed] [Google Scholar]
- Lebon P., Girard S., Thépot F., Chany C. Présence constante d'interféron de type alpha dans les liquides amniotiques humains. C R Seances Acad Sci III. 1981 Jul 6;293(1):69–71. [PubMed] [Google Scholar]
- Lindenmann J., Deuel E., Fanconi S., Haller O. Inborn resistance of mice to myxoviruses: macrophages express phenotype in vitro. J Exp Med. 1978 Feb 1;147(2):531–540. doi: 10.1084/jem.147.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C. A., Gould J. The role of macrophages in mice infected with murine cytomegalovirus. J Gen Virol. 1978 Oct;41(1):143–153. doi: 10.1099/0022-1317-41-1-143. [DOI] [PubMed] [Google Scholar]
- Mintz L., Drew W. L., Hoo R., Finley T. N. Age-dependent resistance of human alveolar macrophages to herpes simplex virus. Infect Immun. 1980 May;28(2):417–420. doi: 10.1128/iai.28.2.417-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Effect of silica on the pathogenic distinction between herpes simplex virus type 1 and 2 hepatitis in mice. Infect Immun. 1977 Aug;17(2):274–277. doi: 10.1128/iai.17.2.274-277.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Genetics of macrophage-controlled resistance to hepatitis induced by herpes simplex virus type 2 in mice. Infect Immun. 1977 Aug;17(2):268–273. doi: 10.1128/iai.17.2.268-273.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Macrophages and age-dependent resistance to hepatitis induced by herpes simplex virus type 2 im mice. Infect Immun. 1978 Jan;19(1):46–50. doi: 10.1128/iai.19.1.46-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J., Brun del Re G., Buerki H., Keller H. U., Hess M. W., Cottier H. Nonspecific acid esterase activity: a criterion for differentiation of T and B lymphocytes in mouse lymph nodes. Eur J Immunol. 1975 Apr;5(4):270–274. doi: 10.1002/eji.1830050411. [DOI] [PubMed] [Google Scholar]
- Pearsall N. N., Weiser R. S. The macrophage in allograft immunity. I. Effects of silica as a specific macrophage toxin. J Reticuloendothel Soc. 1968 Apr;5(2):107–120. [PubMed] [Google Scholar]
- ROBERTS J. A. GROWTH OF VIRULENT AND ATTENUATED ECTROMELIA VIRUS IN CULTURED MACROPHAGES FROM NORMAL AND ECTROMELIAIMMUNE MICE. J Immunol. 1964 Jun;92:837–842. [PubMed] [Google Scholar]
- Rodgers B., Mims C. A. Interaction of influenza virus with mouse macrophages. Infect Immun. 1981 Feb;31(2):751–757. doi: 10.1128/iai.31.2.751-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selgrade M. K., Osborn J. E. Role of macrophages in resistance to murine cytomegalovirus. Infect Immun. 1974 Dec;10(6):1383–1390. doi: 10.1128/iai.10.6.1383-1390.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Tignor G. H. Host cell receptors for two strains of Sindbis virus. Arch Virol. 1980;66(1):11–26. doi: 10.1007/BF01315041. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Woodward J. G., Frelinger J. A. Macrophage antiviral activity: extrinsic versus intrinsic activity. Infect Immun. 1982 May;36(2):672–677. doi: 10.1128/iai.36.2.672-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C. Correlation of persistent mouse hepatitis virus (MHV-3) infection with its effect on mouse macrophage cultures. Arch Virol. 1976;50(4):279–285. doi: 10.1007/BF01317953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Gresser I. Role of interferon in the pathogenesis of viral diseases of mice as demonstrated by the use of anti-interferon serum. V. Protective role in mouse hepatitis virus type 3 infection of susceptible and resistant strains of mice. J Immunol. 1978 May;120(5):1616–1619. [PubMed] [Google Scholar]
- Zawatzky R., Engler H., Kirchner H. Experimental infection of inbred mice with herpes simplex virus. III. Comparison between newborn and adult C57BL/6 mice. J Gen Virol. 1982 May;60(Pt 1):25–29. doi: 10.1099/0022-1317-60-1-25. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]
- Zisman B., Wheelock E. F., Allison A. C. Role of macrophages and antibody in resistance of mice against yellow fever virus. J Immunol. 1971 Jul;107(1):236–243. [PubMed] [Google Scholar]


