Table 4.
Cross-Coupling of 4-Chloroanisole with Various Secondary Aminomethyltrifluoroborates 3a–g
 | ||||
|---|---|---|---|---|
| entry | R | product | yield (%) | |
| 1 | n-Bu |  |
4d | 97 |
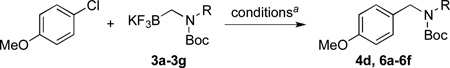
| 2 | i-Pr |  |
6a | 83 |
| 3 |  |
6b | 69 | |
| 4 | 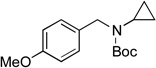 |
6c | 81 | |
| 5 | Bn | 6d | 90 | |
| 6 | 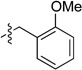 |
 |
6e | 73 |
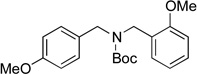
| 7 |  |
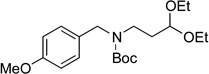 |
6f | 85 |
Reaction conditions: 1.0 equiv of 4-chloroanisole 1.05 equiv of trifluoroborate, 4 mol % of XPhos-Pd-G2 A, 3 equiv of Cs2CO3, toluene/H2O = 4:1 (0.5 M), 85 °C, 3 h.
