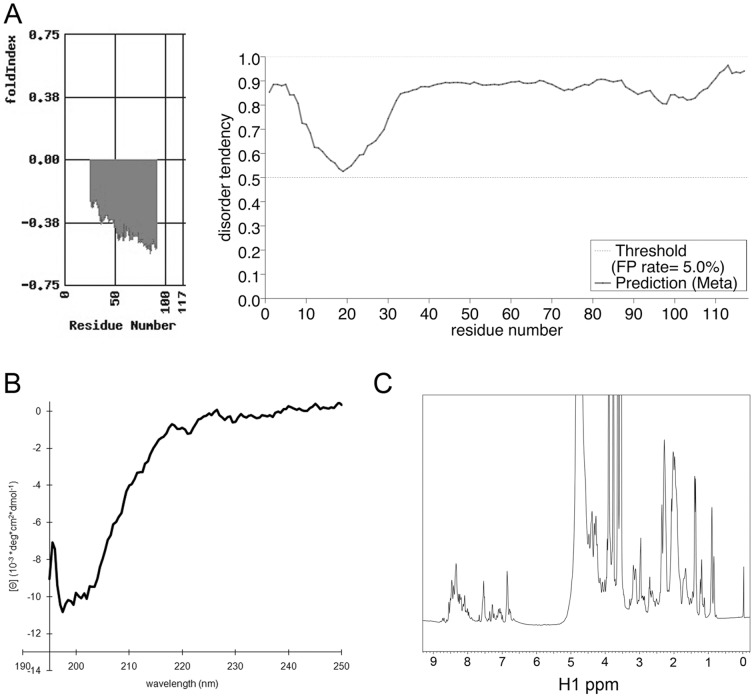
Figure 6. GAGE proteins are intrinsically disordered.
A. Secondary structure and disorder of GAGE-12I predicted by two algorithms: FoldIndex (left panel) and metaPrDOS (right panel). B. Far-UV CD spectrum of GAGE-12I recorded from 195–250 nm of 4.5 µM GAGE-12I in 100 mM NaCl and 50 mM sodium phosphate, pH 5.5 at 25°C. The minimum at around 200 nm, plus the lack of other distinct minima, clearly indicate the protein is predominantly unfolded. C. 1D 1H-NMR spectrum of 4 mg/ml GAGE-12I in 100 mM NaCl, 50 mM sodium phosphate pH 5.5, 0.15 mM DSS and 10% D2O. The very low dispersion of the NMR signals, especially noticeable in the aliphatic region, provides a clear fingerprint of an unfolded protein.